Cervical Cancer
Cervical cancer is a disease in which cells in the cervix grow out of control.
The cervix connects the vagina (birth canal) to the upper part of the uterus (womb).
Anyone with a cervix is at risk for cervical cancer. Most cervical cancers are caused by a long-lasting infection with certain types of HPV. For more information about HPV click here.

Screening Tests and HPV Vaccine
Screening tests like the Pap test and HPV test can help prevent cervical cancer or find it early. The HPV vaccine can also help prevent cervical cancer. For more information about the HPV vaccine click here.
Symptoms
Early on cervical cancer may not cause any signs or symptoms. Later, advanced cervical cancer may cause bleeding or discharge not normal for you and bleeding after sex.
Cervical Cancer Screening Options
The United States Preventative Services Task Force (USPSTF) recommends cervical cancer screening begin at age 21. The table below lists screening recommendations for you. Talk to your doctor about which option is best for you.
USPSTF Screening Recommendation:
- 21-29: Pap tests every three years
- 30-65: Pap test every three years, or Pap test and HPV test every five years, or HPV test alone every five years
- >65: No screening necessary after 2 negative screening tests within the last 10 years and the most recent in the past 3-5 years
Additional Resources:
ERAVE User Application Form
Universal Newborn Hearing Screening, Tracking and Intervention Advisory Board
Act 1559 of 1999 established requirements for birthing hospitals’ newborn hearing screening programs and providers administering a follow-up screening or diagnostic evaluation and created the Universal Newborn Hearing, Screening, Tracking, and Intervention (UNHS) Advisory Board. The UNHS advisory board is designed to provide the state with the information necessary to effectively plan, establish, and evaluate a comprehensive system of appropriate services for children that are at risk for or identified with a hearing condition. The board is composed of seven (7) members appointed by the Governor, with recommendations from the Arkansas Speech-Language-Hearing Association, from the following professions or groups:
- One (1) audiologist
- One (1) audiologist from the Arkansas Department of Health
- One (1) audiologist from Arkansas Children’s Hospital
- One (1) speech-language pathologist
- One (1) pediatrician-neonatologist or ear, nose, and throat physician
- One (1) adult who is deaf or hard of hearing to represent consumer organizations for deaf and hard of hearing persons
- One (1) consumer of services who is a parent of a child or children with hearing loss
As per Act 1559, the Board meets at least semiannually to carry out their business which includes:
- Conducting surveillance to improve access to early hearing detection and intervention services.
- Supporting the Joint Committee on Infant Hearing’s recommendations for early hearing detection and intervention by developing practice recommendations for clinicians statewide.
- Connecting individuals and organizations supporting children that are at risk for or identified with a hearing condition.
- Approving an annual report for the House and Senate Interim Committees on Public Health, Labor, and Welfare which provides information such as, but not limited to, the number of birthing hospitals in compliance with the Act, the number of Deaf or Hard of Hearing children identified, and the availability of follow-up services. The annual report provides a close look at hearing screening activities in birthing hospitals throughout the State of Arkansas, and results of follow-up screening, and outcome measures. Data collected by the Infant Hearing Program each year outlines the strength and weaknesses of the Program; therefore, directing the development and expansion of goals toward improvement.
Board Meetings: The board meets at 3pm on the 4th Tuesday in April and October via Zoom.
Meeting Minutes:
Resources:
- Act 1559 of 1999
- Rules
- Arkansas Department of Health-Infant Hearing Program
- Early Hearing Detection and Intervention Flow Chart
- Early Hearing Detection Roadmap
- Arkansas Academy of Audiology
- Arkansas Academy of Family Physicians
- Arkansas Board of Examiners in Speech-Language Pathology and Audiology
- Arkansas Chapter of the American Academy of Pediatric
- Arkansas Nurses Association
- Arkansas Speech-Language-Hearing Association
- Arkansas Society of Otolaryngology, Head and Neck Surgery
Advisory Board Portal Login – (Board Members Only)
Infant Hearing Program
5800 W. 10th Street, Suite 808
Little Rock, AR 72204
P: 501-280-4740
F: 501-280-4769
Heat Risks
Know the heat-related risks and practice heat safety
Heat stress is a heat-related illness. It happens when your body cannot cool down properly. The body normally cools itself by sweating, but sweating is not enough under certain conditions.
Who is at higher risk?
- Children aged four or younger
- Seniors aged 65 or older
- Anyone overweight
- Anyone who is ill or on certain medications
Heat-related illnesses are preventable
Simple tips to prevent heat stress are:
- Stay in an air-conditioned area during the hottest hours of the day. If your home does not have air conditioning, consider public places like a library, senior center, or mall.
- Wear light, loose-fitting clothing.
- Avoid unnecessary hard work or activities outside or without air conditioning.
- Avoid unnecessary sun exposure. Wear a hat and sunglasses when you are in the sun.
- Drink water often. Don’t wait until you are thirsty.
Common Heat-Related Illnesses:
Heat Stroke: Call 911 right away, move to a cooler place, and help lower the body temperature with cool clothes or a cool bath.
|
Symptoms of Heat Stroke |
||
|
|
|
Heat Exhaustion: Move to a cool place, loosen clothes, put cool, wet clothes on the body, or take a cool bath and sip water. Get medical help immediately if vomiting, dizziness, or symptoms worsen or last longer than one hour.
|
Symptoms of Heat Exhaustion |
||
|
|
|
Heat Cramps: Stop physical activity and move to a cool place, drink water or a sports drink, and wait for cramps to go away before starting more physical activity. Get medical help immediately if someone is on a low-sodium diet, has heart problems, or if cramps last longer than one hour.
|
Symptoms of Heat Cramps |
|
Heat Rash: The best treatment is a cooler, less humid work environment when possible. Keep the rash area dry.
|
Symptoms of Heat Rash |
|
The National Oceanic and Atmospheric Administration (NOAA) and the CDC have a new heat forecast tool called HeatRisk. The HeatRisk Dashboard combines health and temperature data to give a 7-day outlook for hot weather. It uses a 5-level scale to show how risky the heat level in a specific area is.
Knowing the temperature is important to avoid exposure to extreme heat. Extreme heat is when summertime temperatures are much hotter or more humid than average. Humid conditions can make high temperatures more intense, make it harder to stay cool and promote mold growth indoors. Keep indoor humidity levels at 50 percent or below for health and comfort.
Helpful Resources
- National Weather Service Map
- Heath Advisory Map
- Heat Stress Awareness for Workers: OSHA | Fact Sheet
- Extreme Heat: CDC guidance | EPA guidance | American Red Cross | Ready.gov
- Pediatric Vehicular Heatstroke (PVH): Fact Sheet
- Coach Safely Act
- Practice Heat Safety
Backyard Poultry-Associated Salmonellosis
Background
Any backyard poultry can carry Salmonella germs that can make you sick. Always take steps to stay healthy around your flock.
Backyard poultry, such as chickens and ducks, can carry Salmonella germs even if they look healthy and clean. These germs can quickly spread to anything in the areas where the poultry live and roam. You can get sick from touching your backyard poultry or anything in their environment, touching your mouth or food, and swallowing Salmonella germs.
What You Should Do 
- Wash your hands
- Always wash your hands with soap and water immediately after touching backyard poultry, their eggs, or anything in the area where they live and roam.
- Use hand sanitizer if soap and water are not readily available. Consider keeping hand sanitizer at your coop.
- Be safe around backyard flocks
- Don’t kiss or snuggle backyard poultry, and don’t eat or drink around them. This can spread Salmonella germs to your mouth and make you sick.
- Keep your backyard poultry and the supplies you use to care for them (like feed containers and shoes you wear in the coop) outside of the house. You should also clean the supplies outside the house.
- Supervise kids around flocks
- Always supervise children around backyard poultry and make sure they wash their hands properly.
- Don’t let children younger than five years touch chicks, ducklings, or other backyard poultry. Young children are more likely to get sick from germs like Salmonella.
- Handle eggs safely
- Collect eggs often. Eggs that sit in the nest can become dirty or break.
- Throw away cracked eggs. Germs on the shell can more easily enter the egg through a cracked shell.
- Rub off dirt on eggs with a brush, a cloth, or fine sandpaper. Don’t wash eggs because colder water can pull germs into the egg.
- Refrigerate eggs to keep them fresh and slow the growth of germs.
- Cook eggs until both the yolk and white are firm and cook egg dishes to an internal temperature of 160°F to kill all germs.
Call your healthcare provider right away if you have any of these severe symptoms:
- Diarrhea and a fever higher than 102°F
- Diarrhea for more than three days that is not improving
- Bloody diarrhea
- So much vomiting that you cannot keep liquids down
- Signs of dehydration, such as:
- Not peeing much
- Dry mouth and throat
- Feeling dizzy when standing up
Symptoms of Salmonella
- Most people infected with Salmonella experience diarrhea, fever, and stomach cramps.
- Symptoms usually start 6 hours to 6 days after swallowing the bacteria.
- Most people recover without treatment after 4 to 7 days.
- Some people—especially children younger than five years, adults 65 years and older, and people with weakened immune systems—may experience more severe illnesses that require medical treatment or hospitalization.
- For more information about Salmonella, see Salmonella Questions and Answers.
- For more information about Backyard Poultry-Associated Salmonellosis, click here.
Other Resources
- CDC Checklist for Starting a Backyard Flock
- CDC Coops: Episode 1: Doja Chick
- CDC Coops: Episode 2: Chick Inn
- CDC Salmonella Questions and Answers
- FDA: Salmonella and Egg Safety
Medication Recall - Tri-Lo-Sprintec
Teva Pharmaceuticals USA, Inc., has recalled the below lot numbers of the Tri-LoSprintec® 28 Day Regimen for birth control:
- Lot 100038111, Exp. Date: 07 /2024
- Lot 100039678, Exp. Date: 04/2024
- Lot 100042277, Exp. Date: 07/2024
It is still safe to take, but it may not work to prevent a pregnancy as well as it should.
To see if your medicine is part of the recall, check the back of your Ti-Lo-Sprintec package for its lot number and expiration date. If it is not part of the recall, no further action is needed.
If it is part of the recall, you should start using a backup method of birth control (such as condoms) right away until you talk to your health care provider. Please contact your Local Health Unit or other health care provider as soon as possible to schedule a visit to discuss next steps. If you have any unused pills, please bring them to your visit. If you are seeking care from another health care provider, you can still return your unused pills to the Local Health Unit if that is where you got them. When scheduling your visit at the Local Health Unit, please let staff know that your medication is part of the recall.
To find a Local Health Unit near you, please click here.
Coach Safely Act
The Act and the Rule articulate that any association that sponsors or conducts sports training or high-risk youth athletic activities for children aged fourteen (14) and younger shall require all coaches and athletics personnel to complete specific training. The association shall maintain a record of individual course completion for as long as that individual serves as athletic personnel or coach for the association. The courses are to be approved by the Arkansas Department of Health, on these subjects:
- Emergency Preparedness, planning, and rehearsal for traumatic injuries
- Concussions and head trauma
- Heat and extreme weather-related injury familiarization
- Physical conditioning and training equipment usage
- Heart defects and abnormalities leading to sudden cardiac death
Resources:
- Coach Safely Act
- Rules pertaining to Youth Mitigation and Information Courses for Athletics Personnel and Coaches
Approved courses:
Emergency Preparedness, Planning, and Rehearsal for Traumatic Injuries:
- The Collapsed Student: No cost
- First Aid, Health, and Safety: Cost associated with this course
Concussions and Head Trauma:
- Concussions in Sports: No cost
- HEADS UP to Youth Sports (Online Training): No cost
- Bell Ringer: A Concussion Awareness Course for Arkansas Coaches:
- CID: HWB16027
*Note: The courses listed on the Arkansas Ideas portal are available to licensed and non-licensed K-12 educators. - Click here for the course catalog.
- CID: HWB16027
- The Xs and Os of Sports-Related Concussion: Fact Versus Fiction:
- CID: HWG16013
*Note: The courses listed on the Arkansas Ideas portal are available to licensed and non-licensed K-12 educators. - Click here for the course catalog.
- CID: HWG16013
Heat and Extreme Weather-Related Injury Familiarization:
- Heat Illness Prevention: No cost
- Exertional Heat Illness Awareness Training:
- CID: HWG14021
*Note: The courses listed on the Arkansas Ideas portal are available to licensed and non-licensed K-12 educators. - Click here for the course catalog.
- CID: HWG14021
- 108 Degrees: Critical Response
- CID: HWB14022
*Note: The courses listed on the Arkansas Ideas portal are available to licensed and non-licensed K-12 educators. - Click here for the course catalog.
- CID: HWB14022
- The Latest in Heat Illness, Recognition, and Treatment:
- CID: HWG16014
*Note: The courses listed on the Arkansas Ideas portal are available to licensed and non-licensed K-12 educators. - Click here for the course catalog.
- CID: HWG16014
Physical Conditioning and Training Equipment Usage:
- Strength and conditioning: Cost associated with this course.
Heart Defects and Abnormalities Leading to Sudden Cardiac Death:
- Sudden Cardiac Arrest: No cost
- Sudden Cardiac Arrest: When Seconds Count
- CID: HWB19050
*Note: The courses listed on the Arkansas Ideas portal are available to licensed and non-licensed K-12 educators. - Click here for the course catalog.
- CID: HWB19050
Coach Safely
- Coach Safely Foundation: Covering all courses for Coach Safely requirements.
- USA Football: Covering all courses for Coach Safely requirements
- What Athletic Coaches Need to Know
Offered by Terry DeWitt
For inquiries please contact DEWITTT@OBU.EDU
Covering all courses for Coach Safely requirements
*Cost associated with this course
To request review and approval of a course, contact the Arkansas Department of Health Substance Misuse and Injury Prevention Branch:
4815 W. Markham Street, Slot 10
Little Rock, AR 72205
Phone: 1-501-671-1449
Email
Resources:
- Arkansas Activities Association Sports Medicine Information
- Arkansas Activities Association Sports Medicine Fact Sheet for Parents and StudentsArkansas Activities Association Sports Medicine Fact Sheet for Parents and Students
| Office | Address | Phone | Fax |
| Substance Misuse and Injury Prevention | 4815 W. Markham St. - Slot 10 Little Rock, AR 72205 |
501-671-1449 | 501-682-0427 |
Stead Scholars - Student Summer Program
The Stead Scholars Program is a full-time, 8-week summer program (June and July) where the Arkansas Department of Health and the College of Public Health at UAMS mentor undergraduate students enrolled in four-year colleges and universities in Arkansas who are thinking of a career in public health or medicine. The selection of the scholars is made by a review committee composed of the Chief Science Officer, Science Advisory Committee at ADH, as well as Department Heads, the Dean, and Associate Deans at the COPH.
Each student receives a stipend of $4,000 for the 8-week program and is assigned a mentor that they will work with on a project in one of several possible areas concerning public health, either within one of the Centers at ADH or at the UAMS College of Public Health. This is not a clinical program. This experience provides the students with an introduction to public health, exposure to applied and basic public health science and practice, and health policy development. The scholars are expected to attend weekly seminars and meetings held at ADH and COPH where public health topics are presented, discussed, and researched. After their internship, Stead Scholars present a brief PowerPoint presentation to ADH and COPH personnel and leadership on their topic, in conjunction with their mentors.
For the Summer of 2024, registration will open in December and close in mid-February.
- Learn more about Dr. William W. Stead
- Examples of previous projects
- STEAD SCHOLARS – Summer 2024 Registration Information
All Arkansas undergraduates enrolled in accredited four-year colleges and universities in the state are eligible to apply.
All documents are to be submitted by February 16, 2023.
Hansen’s Disease
Hansen’s Disease, often known as Leprosy, is a treatable infectious disease caused by the bacteria M. leprae. The infection is known to attack the nervous system, causing swollen nerves, discoloration and/or inflamed skin, and loss of feeling in affected areas. Without treatment, Hansen’s Disease can cause paralysis of hands and feet, loss of fingers and toes, and blindness, among other symptoms.
Historically, Leprosy was feared as a highly contagious and dangerous disease. However, modern medical science has revealed that Hansen’s Disease is slow to progress and difficult to spread. Transmission requires long exposure times and, once transmission occurs, the disease can take years to manifest any symptoms.
Once detected, Hansen’s Disease can be treated and cured with antibiotics, however, treatment cannot reverse existing symptoms, so it is important to begin treatment as quickly as possible after detection of the disease. It is imperative that you complete your treatment regimen as directed by a physician. Failure to do so can result in the development of antibiotic resistance. There is no vaccine for Hansen’s Disease, however, most people are already naturally immune.
Transmission & Risk Factors
While it is unclear how exactly Hansen’s Disease is transmitted between people, it is evident that the disease does not transmit easily. The disease appears to require extensive close contact over the course of several months or even years to be spread between individuals. It cannot be spread through casual contact; it is entirely safe to shake hands or share a meal with someone with Hansen’s Disease. Further, Hansen’s Disease cannot be spread through sexual contact, and cannot be passed from parent to child during pregnancy. Hansen’s Disease cannot be transmitted at all once treatment has been started.
M. leprae is known to be found naturally in armadillos is the southern United States, and it is suspected that transmission between armadillos and humans can occur, albeit very rarely. While the risk of catching Hansen’s Disease through brief contact with an armadillo is very low, it is best to avoid contact with armadillos when possible.
Hansen’s Disease is very rare in the US, with <200 new cases diagnosed each year nationwide. The disease is somewhat more prevalent in some countries, most notably in Brazil, India, Indonesia, and much of South-Central Africa. Even in these countries however, Hansen’s Disease is still a rare occurrence. In fact, some 95% of people worldwide already have a natural immunity to Hansen’s Disease, rendering it a very rare condition regardless of location.
Symptoms
Hansen’s Disease can cause a variety of symptoms throughout the body, however the most common presentations are found in the skin and the nervous system.
Common skin symptoms include:
- Discoloration of the skin, typically in small patches that may or may not be numb or inflamed
- Abnormal skin growths
- Thick, dry patches of skin
- Ulcers on the soles of feet
- Swelling in the face and earlobes
- Loss of eyebrows or eyelashes
- Burning sensation in the skin
Common symptoms related to nerve damage include:
- Numbness in affected areas
- Muscle weakness and eventual paralysis
- Enlarged/swollen nerves
- Ulcers in the eyes and/or blindness, if facial nerves are affected
- Loss of sense of pain – often leading to unnoticed injuries in affected areas
Other common symptoms include:
- Nasal inflammation
- Nosebleeds
Symptoms of advanced, untreated Hansen’s Disease include:
- Paralysis of hands and feet
- Shortening/loss of fingers and toes
- Chronic, non-healing ulcers on the soles of feet
- Blindness
- Saddle Nose disfigurement
Hansen’s Disease develops very slowly – it can take up to 20 years to develop even minor symptoms. This is because M. leprae reproduces distinctly slowly for an infectious bacterium.
It is imperative to seek treatment as soon as possible after symptoms develop. Hansen’s Disease can be treated and cured relatively painlessly, however symptoms cannot be reversed once they appear.
Testing/Diagnosis
If you develop any of the above symptoms, speak to a doctor immediately. The most common symptom to watch for is patches of discolored skin that have lost sense of touch and/or pain.
Once in contact with a doctor, they will take samples of affected skin or nerves to perform confirmatory tests. These typically involve looking at the sample under a microscope for M. Leprae bacteria or sending the samples to be lab tested for other potential diseases.
Treatment
Once in contact with a doctor, they will take samples of affected skin or nerves to perform confirmatory tests. These typically involve looking at the sample under a microscope for M. Leprae bacteria or sending the samples to be lab tested for other potential diseases.
During treatment:
- Take particular care of areas affected by a loss of sensation; it is very common for people with Hansen’s Disease to experience cuts and burns without even noticing. These injuries can easily become infected or become severe if they aren’t noticed.
- Take the full course of medication as directed by your doctor. Stopping early can result in redevelopment of the illness, emergence of new symptoms, and the development of antibiotic resistance, making the disease harder or even impossible to treat in the future.
- Watch for swollen/inflamed and painful skin patches and nerves and development of a fever, as these can be indications of an adverse reaction to treatment that will require changes and/or additions to treatment medication.
The locations of specialized Hansen’s Disease treatment clinics around the country can be found in the other resources section below. Arkansas is served by the Springdale Hansen’s Disease Clinic:
Springdale HD Clinic
Joseph H. Bates Outreach Clinic of Washington County
614 E. Emma Avenue, Suite 247
Springdale, AR 72764
PH: (479)-751-3630
Fax: (479)-751-4838
Physician: Linda McGhee, M.D.
Public Health Nurse: Ruby Lewis, R.N.
Telephone: (479) 841-5779
Other Resources:
Much of the information on this page is taken from the following sources. You can follow the links below to find out more:
Links to care:
- Hansen’s Disease clinics in the United States
- National Hansen’s Disease Program Fact Sheet and Contact Information
Additional information:
Naegleria Fowleri
Naegleria fowleri is an ameba (single-celled living organism) that lives in soil and warm freshwater, such as lakes, rivers, and hot springs. It is commonly called the “brain-eating ameba” because it can cause a brain infection when water containing the ameba goes up the nose. Only about three people in the United States get infected yearly, but these infections are usually fatal.
You cannot get infected from drinking water contaminated with Naegleria. You can only be infected when contaminated water goes up into your nose.
Symptoms usually start with severe headache, fever, nausea, and vomiting and progress to stiff neck, seizures, altered mental status, hallucinations, and coma.
Naegleria fowleri infects people when water containing the ameba enters the body through the nose. This typically happens when people go swimming, diving, or when they put their heads under fresh water, like in lakes and rivers. The ameba then travels up the nose to the brain, where it destroys the brain tissue and causes a devastating infection called primary amebic meningoencephalitis (PAM). PAM is almost always fatal.
Naegleria fowleri infections may also happen when people use contaminated tap water to rinse their sinuses by sending water up the nose. In very rare instances, people have gotten Naegleria fowleri infections from recreational water that didn’t have enough chlorine in it, such as pools, splash pads, or surf parks. There is no evidence that Naegleria fowleri can spread through water vapor or aerosol droplets (such as shower mist or vapor from a humidifier).
Recommended preventative steps include:
- Avoid jumping or diving into bodies of warm fresh water, especially during the summer.
- Hold your nose shut, use nose clips, or keep your head above water when in bodies of warm fresh water.
- Avoid putting your head under water in hot springs and other untreated geothermal waters.
- Avoid digging in, or stirring up, the sediment in shallow, warm fresh water. The amoebae are more likely to live in sediment at the bottom of lakes, ponds, and rivers.
Resources:
Other Uniformed Service Members, Veterans, and their Spouses
The Arkansas Act creates two licensing pathways for veterans and their spouses, automatic or expedited.
Who does it apply to?
The Arkansas law applies to:
- Uniformed Service Members that the SCRA does not cover.
- Uniformed Service Veteran who resides in or establish residency in Arkansas.
- The spouse of a Uniformed Service Member that the SCRA does not cover.
- The spouse of a Uniformed Service Veteran who resides in or establish residency in Arkansas.
- The spouse of a Uniformed Service Member who is assigned a tour of duty that excludes the uniformed service member's spouse from accompanying the uniformed service member and the spouse relocates to this state;
- A uniformed service member who is killed or succumbs to his or her injuries or illness in the line of duty if the spouse establishes residency in the state.
Automatic Licensure:
Certain licensing entities provide automatic licensure under state law. The individual must contact one of the listed licensing functions and:
- the individual is a holder in good standing of occupational licensure with similar scope of practice issued by another state, territory, or district of the United States; and
- the individual pays the applicable licensure fee.
Quick Links:
- Nursing Board
- Board of Optometry
- Medical Board
- Physical Therapy Board
- Board of Dental Examiners
- Pharmacy Board
- Board of Dental Examiners
- Athletic Training Board
- Acupuncture & Related Techniques Board
- Board of Examiners in Counseling/MFT
- Board of Podiatric Medicine
- Dietetics Licensing Board
- Psychology Board
- Board of Examiners in Speech-Language Pathology and Audiology
- Board of Examiners of Alcoholism & Drug Abuse Counselors
- Board of Dispensing Opticians
- Board of Chiropractic Examiners
- Social Work Licensing Board
- Board of Hearing Instrument Dispensers
- Massage Therapy
- Cosmetology and Body Art
- Radiologic Tech
- Lay Midwife
- Drinking Water Operator
- Sanitarians
- Plumbers
- Orthotist, Prosthetists, and Pedorthists
- Lead-Based Paint
- Emergency Medical Services (EMS)
Expedited Licensure:
Arkansas law does allow licensing entities to submit rules for an expedited process versus an automatic process. The boards and commissions below have expedited requirements.
- Medical Board
- Physical Therapy Board
- Board of Dental Examiners
- Pharmacy Board
- Board of Dental Examiners
Active-Duty Military and their Spouses
The new SCRA provision allows servicemembers and their spouses to use their professional licenses and certificates in certain circumstances when they must relocate due to military orders. For a license to be considered valid in a new location, a servicemember or their spouse must satisfy the following five criteria:
- Have moved to a location outside the jurisdiction of the licensing authority that issued the covered license or certificate because of orders for military service;
- Provide a copy of the military orders to the licensing authority in the new jurisdiction;
- Have actively used the license or certificate during the two years immediately preceding the move;
- Remain in good standing with:
- the licensing authority that issued the covered license or certificate; and
- every other licensing authority that issued a license or certificate valid for a similar scope of practice and in the discipline applied for in the new jurisdiction; and
- Submit to the authority of the licensing authority in the new jurisdiction for the purposes of standards of practice, discipline, and fulfillment of any continuing education requirements.
Active-duty members and their spouses should use the links below to find contact information for the appropriate licensing entity.
Quick Links:
- Nursing Board
- Board of Optometry
- Medical Board
- Physical Therapy Board
- Board of Dental Examiners
- Pharmacy Board
- Board of Dental Examiners
- Athletic Training Board
- Acupuncture & Related Techniques Board
- Board of Examiners in Counseling/MFT
- Board of Podiatric Medicine
- Dietetics Licensing Board
- Psychology Board
- Board of Examiners in Speech-Language Pathology and Audiology
- Board of Examiners of Alcoholism & Drug Abuse Counselors
- Board of Dispensing Opticians
- Board of Chiropractic Examiners
- Social Work Licensing Board
- Board of Hearing Instrument Dispensers
- Massage Therapy
- Cosmetology and Body Art
- Radiologic Tech
- Lay Midwife
- Drinking Water Operator
- Sanitarians
- Plumbers
- Orthotist, Prosthetists, and Pedorthists
- Lead-Based Paint
- Emergency Medical Services (EMS)
Licensure Reciprocity for Active Duty, Veterans, and Spouses
Two laws apply to the occupational licensure of current and former military members and their spouses, the Servicemembers Civil Relief Act (SCRA) and the Arkansas Occupational Licensing of Uniformed Service Members, Veterans, and Spouses Act of 2021. For active duty and their spouses, the federal law is controlling but because it does not extend to veterans and their spouses, the state law controls requirements for those individuals.
Quick Links
Active-Duty Military and their Spouses:
On January 5, 2023, the SCRA was amended to provide a way for those who have a PCS to move to carry their license with them to their new duty station. This reduces the burdens associated with interstate military moves and allows military spouses to obtain meaningful employment more quickly.
Other Uniformed Service Members, Veterans, and their Spouses:
The act is intended to help uniformed service member, veterans, and their spouses by removing barriers that impede the launch and sustainability of civilian occupational careers and employment faced by uniformed service members, uniformed service veterans, and their spouses. Under the Act “Uniformed Service Members” include those also covered under the SCRA. “Uniformed Service Veterans” are defined as former members of the United States uniformed services discharged under conditions other than dishonorable.
Body Art Forms
- Act 990 Prelicensure Petition
- Artist Instructor
- Body Art Relocation
- Certification of Record
- Event Sponsor – Temporary Demonstration License
- Examination & Initial Licensure Application
- New Establishment Form
- New Institution Form
- Reciprocity Requirements
- Reinstatement Form
- Retake Examination Application
- Student Artist Application
- Temporary Demonstration License – Arkansas Guest Artist
- Temporary Demonstration License – Out of State Guest Artist
Medical Ionizing Radiation Licensure Committee (MIRLC)
Interested applicants may fill out the Online Application for Appointment for consideration.
The Medical Ionizing Radiation Licensure Committee shall be an advisory committee to the State Board of Health and shall consist of ten (10) members, as follows: Nine (9) members to be appointed by the Governor; and One (1) member shall be the Secretary of the Department of Health or his or her designee.
Among the committee members shall be:
- two (2) radiologic technologists
- one (1) nuclear medicine technologist
- one (1) radiation therapist
- two (2) radiation practitioners
- one (1) licensed practitioner
- one (1) radiation health/medical physicist
- one (1) consumer
The members shall be appointed for three-year staggered terms to be assigned by lot. Committee members shall serve until replaced. The committee shall meet at least quarterly. The MIRLC reviews allegations and violations, may conduct disciplinary proceedings, and issue subpoenas as provided in the act.
| Membership: 2021-2023 | |
| Shane David, ADH Representative | Radiation Control Branch Chief |
| Tracy McKnight | Radiologic Technologist |
| Adriane Travis | Radiologic Technologist |
| Lisa Rhoden, Committee Chair | Nuclear Medicine Technologist |
| Sheila Randolph | Radiation Therapist |
| Rogerich Paylor, M.D. | Radiation Practitioner |
| Richard Nelson, M.D. | Radiation Practitioner |
| Allan Kirkland M.D. | Licensed Practitioner |
| Paul Bruce, MS, DABR | Medical Physicist |
| vacant | Consumer |
Meeting Schedule 2024
- April 30
- July 9
- October 8
Meeting Minutes
Click here for the Radiologic Technology Licensure Program homepage.
WIC Acceptable Use Privacy Policy
my Arkansas WIC Program
Your privacy is very important to us. The my Arkansas WIC App collects basic information during the initial registration process to receive messages. The app does not store any personal information collected. By using the app, you are accepting the practices described in this privacy policy.
Information Collected
A State WIC ID and Household ID are collected during the initial registration to receive messages. This information is stored in a database on secure servers and it is not shared with third parties. This information is shared with the WIC Program to report app statistics. The app uses the camera to capture Universal Product Codes (UPCs) to verify if a scanned product is WIC approved. All the information retrieved from the servers is secured during transmission using HTTPS.
Phone Permission
Messages display the WIC clinic phone number. The app uses the Phone permission to automatically call the number when selected.
Photos/Media/Files Permission
To scan the UPCs, the app uses the camera plugin which requires the Photos/Media/Files permission.
Network Connection Permission
To validate the connectivity of the device, the app uses Network Connection permission to access the network.
Location Permission
Used by Store Locator function to determine the closest stores.
Policy Modifications
This privacy policy may be changed without notice. Any changes will be posted at this location. We recommend checking this policy periodically so you are fully aware of any changes.
HPV Vaccine
HPV Vaccine is Cancer Prevention
What is HPV Vaccine?
HPV vaccine protects against infection with the Human Papillomavirus (HPV), which can cause several types of cancer in both men and women. The vaccine also prevents most genital warts and cervical precancers. Gardasil 9, which protects against 9 HPV types, is the only HPV vaccine currently in use in the United States.
What Cancers Does HPV Cause?
HPV can cause oropharyngeal (back of mouth and throat), cervical, penile, anal, and vulvar cancers. HPV is estimated to cause nearly 36,500 cases of cancer in men and women every year in the United States. HPV vaccination can prevent 33,700 of these cancers by preventing the infections that cause them.
Who Should Get HPV Vaccine? When Should They Get It?
All children age 9-12 years, both boys and girls, should get two doses of HPV Vaccine, given 6-12 months apart.

Early protection works best. That’s why HPV vaccine is recommended earlier rather than later, since it protects children best when given before they ever have contact with the virus.
Teens and young adults should be vaccinated too. Everyone through age 26 years should get HPV vaccine if they were not fully vaccinated already.
Some adults age 27 through 45 years who were not already vaccinated might choose to get HPV vaccine after speaking with their doctor about their risk for new HPV infections and the possible benefits of vaccination for them.
How Well Do These Vaccines Work?
HPV vaccination works extremely well.
- Since HPV vaccination was first recommended in 2006, infections with HPV types that cause most HPV cancers and genital warts have dropped 88% among teen girls and 81% among young adult women.
- Fewer teens and young adults are getting genital warts.
- HPV vaccination has also reduced the number of cases of precancers of the cervix in young women.
- The protection provided by HPV vaccines lasts a long time. People who received HPV vaccines were followed for at least about 12 years, and their protection against HPV has remained high with no evidence of decreasing over time.
Is the HPV Vaccine Safe?
HPV Vaccine is very safe. Over 15 years of monitoring have shown that HPV Vaccines are very safe and effective. Like all vaccines, scientists continue to monitor vaccines to ensure they are safe and effective.
Where Can I Find These Vaccines?
HPV Vaccine is available in many doctor's offices and clinics, especially those that take care of children and young adults. Many pharmacies also vaccinate children and adults for HPV. In addition, all of ADH’s Local Health Units (LHUs) give HPV vaccinations to all children and some adults.
How Do I Pay for These Vaccines?
Most health insurance plans cover routine vaccinations like HPV vaccine. The Vaccines For Children (VFC) program also provides vaccines for children 18 years and younger who are uninsured, underinsured, Medicaid-eligible, American Indian, or Alaska Native. In AR many clinics that care for children, as well as some pharmacies, are VFC providers. In addition, all of ADH’s Local Health Units (LHUs) are VFC providers.
Resources:
HPV Prevention
What is HPV?
HPV or Human Papillomavirus is the most common sexually transmitted disease (STD). HPV is a different virus than HIV and HSV (herpes). HPV is so common that nearly all sexually active men and women get it at some point in their lives. There are many different types of HPV. Some types can cause health problems including genital warts and cancers. But there are vaccines that can stop these health problems from happening.
Signs and Symptoms
- Most people have no symptoms
- Develop within weeks or months after exposure, or not at all
- Soft fleshy lumps on or near genitals or anus
- Itching or burning around genitals
- Warts may be hidden in the vagina or anus
- The warts may go away with treatment, but the HPV infection can persist
- In 90% of cases, HPV disappears spontaneously within 2 years after infection
Transmission
Genital Warts are spread by:
- Vaginal sex
- Oral sex (rare)
- Anal sex
- Contact with infected person’s warts
- Infected mother to newborn (very rare)
- Warts/HPV may be spread even if no warts are visible because the virus may be present on areas not protected by a condom
Complications
If left untreated, Genital Warts can:
- Spread to sex partners
- Be passed to newborn during childbirth; can cause warts in infant’s throat (very rare)
Some virus strains lead to abnormal Pap tests and increased risk of cervical cancer, but these strains do not cause visible warts. Sexually active women should have yearly Pap tests starting 3 years after they first had sex. HPV may also play a role in cancers of the anus, mouth/throat, penis, and vagina.
A physician may perform a special test to identify the cancer-associated strains.
Prevention
- Gardasil-9 is the vaccine available for males and females age 9-26 years of age to protect against the types of HPV that cause most cases of cancer and genital warts.
- HPV vaccination is also recommended for some people age 27-45 years of age if they are not already vaccinated. People in this age range should speak to their doctor about the benefits of HPV vaccine for them.
- The surest way to avoid transmission of sexually transmitted diseases is to abstain from sexual contact or to be in a long-term mutually monogamous relationship with a partner who has been tested and is known to be uninfected.
- Latex condoms, when used consistently and correctly, can reduce the risk of transmission only when the infected areas are covered or protected by the condom.
- Use a new latex condom properly for any sexual contact.
- Limit the number of sex partners.
Testing and Treatment
- Get an exam from a medical provider if infection is suspected.
- Warts can be treated, but HPV cannot be cured.
- HPV requires medical treatment by doctor.
- Drugstore treatment for other kinds of warts may be harmful if used on genital warts.
“High risk” HPV strains that cause cancer do not cause visible genital warts. But, high-risk strains may be present along with visible warts.
The body may eventually clear the virus with or without treatment.
The HPV vaccine that is licensed by the FDA, recommended by CDC and currently distributed in the USA is Gardasil-9 (made by Merck).
Infection Preventionist (IP)/ICAR
The Arkansas Department of Health would like to introduce a free, confidential, and non-regulatory resource for your team. The ADH HAI program has three certified infection control (CIC) infection prevention nurses (IPs), housed across the state who would like to come and discuss infection prevention strategies and assist with addressing any identified gaps to help guide quality improvement activities within your facility. The IPs will work with you and your team to review and meet Center of Disease Control and Prevention (CDC) best practices.
If you or your team would like to participate in an assessment, click the link here to request an ICAR visit and, ideally, you will receive communication regarding your request within two business days.
| Office | Address | Phone |
| Healthcare-Associated Infections | 4815 W. Markham St. - Slot 42 Little Rock, AR |
501-280-4368 |
Ambulance Service Applications
Please download and submit the applications with applicable fees to the Section of EMS:
ASBP - Complaints
ASCC Programs & Services
Quick Links:
- Who is eligible?
- Make a referral
- Case management services
- Equipment loan program
- Attendant Care
- Purchased services
- Spinal Disorders Camp
- Spinal Cord Disability Registry
- Education and Training
- Spinal Connection
Who is Eligible for Services from Arkansas Spinal Cord Commission?
The Arkansas Spinal Cord Commission serves only Arkansas residents who have sustained a spinal cord disability.
- The individual must be an Arkansas resident at the time of referral.
- The individual may be of any age, ASCC provides services to individuals with spinal cord disabilities from birth to death.
- The disability must be severe enough to meet the agency’s medical criteria.
Arkansas state law (ACA 20-8-206requires that: Every public and private health and social agency and attending physician shall report to the commission within five (5) calendar days after identification of any spinal cord disabled person…. Within fifteen (15) days of the report and identification of a spinal cord-disabled person, the commission shall notify the spinal cord-disabled person or the most immediate family members of their right to assistance from the state, the services available, and the eligibility requirements.
If a healthcare professional, individual with a spinal cord disability or another interested individual identifies someone with a spinal cord disability, contact the Arkansas Spinal Cord Commission to make a referral. It is better to refer someone who is not eligible than not to refer someone who is eligible for services.
To make a referral, contact the regional Case Management office or call the ASCC administrative office at 501-296-1788 or 800-459-1517 between 8am and 4:30pm. Monday - Friday. Confidential fax is available 24 hours a day at 501-296-1787. You can find the ASCC Referral Form here.
Damage to the Spinal Cord
Spinal cord disabilities result from damage to the spinal cord. ASCC serves only Arkansans whose spinal cord has been damaged. This damage may result from a traumatic spinal cord injury, from a birth defect such as spina bifida or sacral agenesis, or a disease process of the spinal cord including spinal cord tumors, amyotrophic lateral sclerosis, multiple sclerosis, or spinal stenosis. This list of diagnoses is not exhaustive and if a health care provider or individual has an individual whom they believe may qualify for services, that individual should be referred and an ASCC Case Manager will be assigned to collect medical records and information to determine the spinal cord disability. Individuals with disabilities of the spinal column that do not affect the spinal cord, the brain or brain stem (only without cord involvement) or the peripheral nerves typically do not qualify for ASCC services.
Medical Criteria
Once an individual is determined to have a spinal cord disability, the ASCC Medical criteria are applied. The medical criteria assess the actually disability or limitations in function resulting from the spinal cord damage. For the purpose of determining disability, the four criteria listed below are used. An individual must have at least three of the following symptoms. Medical records or information will be used to verify these criteria:
- Lack of Normal Motor Control / Paralysis - A lack of normal voluntary motor function. There should be enough weakness and/or spasticity to significantly interfere with normal self-care activities and/or mobility. In most cases, this will require the use of a wheelchair, scooter, walker, braces (including AFOs, KAFOS), crutches, or cane for mobility. In some cases, particularly those with a diagnosis of central cord syndrome, the paralysis may be more severe in the arms and hands than in the legs to the point that adaptive devices or assistance is required for completing activities of daily living such as feeding, dressing, and hygiene.
- Lack of Normal Sensation - A lack of normal sensation at or below the level of lesion that results in absent or impaired ability to discern touch, pressure, pain (the ability to tell sharp from dull), or temperature (the ability to tell hot from cold). The individual should have enough loss of sensation to have more than normal risk to the skin and musculoskeletal structures.
- Loss of Normal Bladder Control - The lack of ability to voluntarily empty the bladder in a timely manner without accidents or the use of equipment or medication. Frequently, individuals with spinal cord injury will present with a neurogenic bladder. When this condition is present, it is usually obvious with symptoms of either urinary incontinence (involuntary voiding) or urinary retention (inability to void). However, with minimal spinal cord damage, the symptoms may be subtler.
- Loss of Normal Bowel Control - The lack of ability to voluntarily empty the bowel in a timely manner without accidents or use of equipment or medication. Frequently, individuals with damage to the spinal cord will present with a neurogenic bowel.
For additional information regarding the ASCC Medical Criteria, please see the Arkansas Spinal Cord Commission Policies and Procedures Manual policy CS-3 – Medical Eligibility Criteria.
Financial Criteria
The Arkansas Spinal Cord Commission and our Case Managers provide numerous services to individuals who have met the medical criteria, including life-long case management, counseling, guidance, advocacy, resource identification, referral for other services, technical assistance, and other services without regard to income.
When an individual needs purchased services or financial assistance in obtaining equipment or services related specifically to the spinal cord disability, the individual must also meet financial criteria.
In order for ASCC to assist a client with purchased services:
- The individual must complete a Financial Resource Assessment with the Case Manager that includes information about earned and unearned income, savings, assets, and spinal cord disability-related expenses.
- There must be no other resource (insurance, Medicare, Medicaid, personal savings, etc) available to assist with the purchase. An ASCC Financial Eligibility Assessment has been completed no more than 1 year from the date of purchase.
- The expenditure or service must be related to the spinal cord disability and a covered service under ASCC procedures. The Commission has specific guidelines and limits on some services.ASCC follows Arkansas State Procurement laws in all purchasing.
- ASCC does not make purchases related to normal living requirements including but not limited to; housing, utilities, food, clothing, vehicles, or medical insurance payments. The Commission is precluded from making any van modifications.
- The Commission is precluded from making any payment for any hospitalization.
For additional information regarding the ASCC Financial Criteria please see ASCC Case Management Procedures Manual, Chapter 5, Determining Financial Eligibility
For additional information regarding the procedures and array of services purchased by ASCC, please see ASCC Case Management Procedures Manual, Chapter 10, and Purchasing Services for your Client.
Please note, the ASCC budget for purchased services is small and the agency may not be able to make purchases to meet all of the client’s needs due to these budgetary constraints.
Arkansas Volunteer Health Care Act
The purpose of the Arkansas Volunteer Health Care Act is:
- To ensure compliance with Ark. Code Ann. §16-6-201, Ark. Code Ann. §20-8-801, et seq., and Ark. Code Ann. §17-95-106;
- To provide for the registration of Free or Low-Cost Health Care Clinics, as defined in Ark. Code Ann. §16-6-201 and herein, under the Arkansas Volunteer Immunity Act, to provide immunity from civil damages to physicians and licensed healthcare professionals who are rendering free and voluntary professional health care services;
- To provide for the registration of healthcare providers and medical professionals, as defined in Ark. Code Ann. §20-8-803, under the Volunteer Health Care Act, who are providing Volunteer Healthcare Services to low-income patients to provide immunity from civil damages to medical professionals who contract with the Department of Health; and,
- To provide for the registration of retired physicians and surgeons who are licensed to practice medicine by the Arkansas State Medical Board under the laws of the State of Arkansas and who are providing Volunteer Healthcare Services to provide immunity from civil damages, under Ark. Code Ann. §17-95-106.
The Arkansas State Board of Health is responsible for the registration of healthcare professionals, healthcare providers; clinics pursuant to Act 276 of 1997, Act 958 of 2017, and Act 968 of 2021.
The links below are available for online registrations:
- Arkansas Volunteer Health Care Act Retired Physician/Surgeon Application
- Arkansas Volunteer Health Care Act Medical Professional/Student Application
- Arkansas Volunteer Health Care Act Health Care Provider Application
Resources:
Avian Influenza H5N1 Virus (Bird Flu)
Background
Avian influenza viruses usually infect birds, but rare cases of human infection with these viruses have been reported. Humans that get avian influenza usually have come in direct contact with infected birds, birds that have died from avian influenza, or bird droppings from infected birds. "Bird flu" viruses have been found in many other species of animals, including mammals on land and in the water. These viruses can have a variety of presenting symptoms in these different species, and can cause mild to severe illness, even death in certain circumstances.
There are two categories of influenza A viruses in birds: highly pathogenic and low pathogenic, which refers to their ability to cause very mild to very severe illness in birds, specifically poultry (chickens). These bird flu virus strains typically infect waterfowl, such as ducks, and rarely infected domestic birds, including chickens, in the past. However, since February 2022, the United States has been experiencing an outbreak of a highly pathogenic avian influenza (HPAI) A(H5N1) strain in both domestic and wild birds, making this the largest animal health event to ever affect the U.S. Additionally, since March 2024, this virus has been detected in dairy cattle in several states, which exposed and even infected a small number of dairy farm workers, all of whom experienced mild symptoms and have since recovered.
For more information about “bird flu” in humans, please visit the CDC website.
It is important to remember that HPAI is primarily a production and economic concern for our poultry industry. It is safe to consume properly handled and cooked poultry products, including meat and eggs.
Current US Situation as of July 17, 2024
There is ongoing surveillance throughout the US and the world to look for "bird flu" in migratory waterfowl. In the US, the US Department of Agriculture, the US Department of the Interior, and the US Department of Health and Human Services work together on this surveillance. More information on surveillance and positive results in domestic and wild animals can be found on the USDA website.
The Arkansas Department of Health works closely with other state partners, including the Arkansas Department of Agriculture and the Arkansas Game and Fish Commission, on all animal and human-related public health concerns.
Other Resources
- Arkansas Department of Agriculture information on Avian Influenza
- University of Arkansas Cooperative Extension Service information on Avian Influenza
- CDC information on Avian Influenza (Bird Flu)
- USDA APHIS information on Avian Influenza
- Arkansas Game and Fish Commission information on Avian Influenza
- Poultry owners should monitor their flocks and report any possible symptoms to the Arkansas Department of Agriculture at 501-823-1746. For wild birds, please use the Arkansas Game and Fish Commission online tool to report sick or dead birds.
- Arkansas Department of Health Avian Influenza Prevention Measures and Post-Exposure Monitoring Instructions
What is avian influenza (bird flu)?
Avian influenza, also called bird flu, is caused by a virus that infects birds such as chickens, turkeys, geese, pigeons, and pheasants. The virus is found in an infected bird’s poop as well as fluids from the bird’s eyes, nose, or mouth.
Bird flu doesn’t usually infect people, however, there are a few ways you can get infected. A person can be infected with bird flu if they:
- Touch their eyes, nose or mouth after working with infected live or dead birds.
- Touch their eyes, nose, or mouth after visiting places where infected birds have lived.
- Breathe in droplets or dust contaminated with the virus.
- Have close contact with a person already sick with the disease, though this is rare.
Symptoms of bird flu range from mild eye infections to a flu-like illness. In severe cases, bird flu can cause pneumonia and death.
Who can get bird flu?
Most humans are unlikely to get bird flu. Individuals who work with animals, such as veterinarians, farmers, animal industry experts, and wildlife professionals, or people who visit poultry farms or live-animal markets may be more likely to get infected.
What can people do to prevent bird flu?
There is no vaccine to prevent bird flu, but there are steps you can take to protect yourself.
- Avoid touching birds and visiting places where birds live
- Do not touch birds whether they are alive or dead.
- Avoid visiting live bird and poultry markets.
- Avoid Germs
- Wash your hands often with soap and water for at least 20 seconds.
- If soap and water aren’t available, clean hands with hand sanitizer containing at least 60% alcohol.
- Don’t touch your eyes, nose, or mouth. If you need to touch your face, make sure your hands are clean.
- Cover your mouth and nose with a tissue or your sleeve (not your hands) when coughing or sneezing.
- Try to avoid close contact, such as kissing, hugging or sharing eating utensils or cups with people who are sick.
If you feel sick and think you may have bird flu, particularly if you have a fever, talk to a healthcare provider and tell them about any contact with birds. Stay away from other people while are sick.
Preparing Food
The Arkansas poultry industry maintains rigorous health and safety standards, including routine monitoring for avian influenza. It is safe to eat properly handled and cooked poultry; there is no concern of avian influenza risk in processed poultry products.
Always properly handle and cook poultry products:
- Clean: Wash hands and surfaces often.
- Separate: Separate raw meats and poultry from other foods.
- Cook: Cook all poultry to 165°F.
- Chill: Refrigerate promptly.
For more information, click here
AMPOQRC Resources
Reports:
State Resources:
- Arkansas Department of Health Maternity Program
- Arkansas Department of Health WIC Program
- Arkansas Maternal Mortality Review Committee
- UAMS Institute for Digital Health & Innovation High-Risk Pregnancy Program formerly called “ANGELS”
- Arkansas Department of Human Services –Division of Children and Family Services
National Resources:
National Organizations:
- American Academy of Pediatrics (APP)
- American Congress of Obstetricians and Gynecologists (ACOG)
- Association of Maternal and Child Health Programs (AMCHP)
- Association of Women’s Health, Obstetric, and Neonatal Nurses (AWOHN)
- CityMATCH
Arkansas Maternal and Perinatal Outcomes Quality Review Committee
The Arkansas Maternal and Perinatal Outcomes Quality Review Committee started with Act 1032 of 92nd General Assembly Regular Session, 2019. The committee is charged with reviewing data on births and developing strategies for improving birth outcomes.
Purpose: The purpose of the Arkansas MPOQRC is to ensure that the maternal and perinatal levels of care classification systems are implemented and maintained in the state of Arkansas with the goal of decreasing maternal and infant morbidity and mortality.
Mission: Ensuring the best health outcomes for mothers and newborns.
Vision: all Arkansas families have access to high-quality care for their family members to achieve optimal long-term health and wellness.
Goals:
- Create a continuous quality improvement process that includes but is not limited to:
- Reviewing maternal and neonatal data from labor and delivery units, nurseries, and neonatal intensive care units in the state
- Sharing of aggregate data with the committee aligned with improvement efforts
- Using comparative data (where it exists) to assess opportunities as well as the success of hospitals in the state
- Identify agreed-upon outcomes and process measures
- Develop aims and interventions to reduce maternal and infant mortality and morbidity
- Review self-verification data and quality data collection tool information yearly for all participating facilities
- In addition to yearly self-verification, the Committee will conduct facility site reviews for level III-A, III-B, and IV facilities every three years
- Provide education to the committee based on current evidence and state needs
- Advocacy and policy recommendations
- Delegation of the above goals and objectives to already existing collaboratives in the state of Arkansas as voted on by the committee
- Reviewing maternal and neonatal data from labor and delivery units, nurseries, and neonatal intensive care units in the state
Reports
For questions, please contact ADH MPOQRC.
Project Firstline
What is Project Firstline?
Project Firstline is a nationwide collaboration led by the Centers for Disease Control and Prevention (CDC) to provide infection control training and education to frontline healthcare workers.
The safety of our healthcare workers is our top priority. The Arkansas Department of Health Healthcare-Associated Infections (HAI) program and Project Firstline are designed to work directly with frontline healthcare workers to provide knowledge, through free training, and access to resources. The ADH Project Firstline program is here to help ensure that all healthcare workers will be able to adapt and understand infection control principles, protocols, and procedures to protect our community, ourselves, and everyone around to help keep Arkansans healthier and safer.
Your Resource for Infection Control
Infection Prevention and Control are critical for preventing the transmission of infectious diseases in all healthcare settings. Project Firstline is committed to helping anyone working in a healthcare facility to help protect themselves, the community, and their patients from infectious diseases. Preventing germs from spreading in healthcare facilities is most effective when everyone uses infection control practices in a consistent manner.
- To get a better understanding of infection control so you can keep yourself and others safe, click here.
Explore Project Firstline Training & Education Videos
Training is a key factor of Project Firstline. Regardless of your role or previous training or education, healthcare workers will gain important information from this training that could save your life as well as others.
Here are some of the topics you will learn during the training:
- Personal Protective Equipment (PPE)
- How COVID-19 spreads
- Source Control
- Triage and Screening
- Environmental Cleaning
- Virus Strains
- Multi-dose vials
- Ventilation.
Click here for more information.
Learning Needs Assessment
We invite all healthcare workers in Arkansas to give us information to develop resources to meet infection prevention training needs. The link below will take you to an assessment. Responses are confidential and will be aggregated to inform decisions, development, and delivery of future training to ensure it best meets the needs of you and your colleagues.
Please complete our Learning Needs Assessment.
Register for Webinars
Want to get access to the newest Project Firstline resources? Please provide your contact information at the below link so that we can contact you about the availability of new resources and access to future webinars. The goal of the webinar is to keep you updated on new information as well as any training or information you might have already missed.
Questions? Contact us at adh.projectfirstline@arkansas.gov.
Mpox
Symptoms | Transmission | Prevention | Testing | Treatment |
Vaccines | Vaccine Location Map
Mpox call-line: 1-800-803-7847
The first case of Mpox in Arkansas was identified in July 2022.
| Cases of Mpox in Arkansas | |
|---|---|
| 82 cases among Arkansas residents (last new case 1/9/2024) |
*For Mpox case data and vaccine locations, click here to visit the data hub*
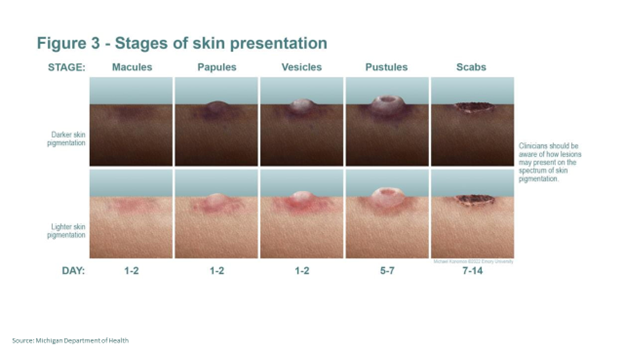
The symptoms of Mpox include fever, headache, muscle pain, and a painful rash that occurs seven to 14 days after exposure. The rash may be located on or near the genitals or anus but could also be on other areas like the hands, feet, chest, or face
Mpox is spread through close contact and can be transmitted to anyone regardless of age, gender, sexual orientation, race, and ethnicity. It can be spread by direct skin-to-skin contact with infectious rash, scabs, or body fluids. This can include household and/or intimate contact. Spreading can also occur when contacting contaminated items, such as clothing. It may be transmitted through contact with respiratory secretions; however, it is not an airborne illness.
Mpox is NOT spread through casual, brief conversations or walking by someone with Mpox, like at a grocery store.

Contact a healthcare provider right away to talk about diagnosis, testing, and treatment options. If you do not have a health care provider, please contact the ADH call center at 1-800-803-7847 to locate a local health unit near you.
Antiviral drugs are also available for the treatment of the illness. Although most cases are self-limiting without treatment, you might need treatment if you have severe disease, are immunocompromised, have a history of atopic dermatitis or other active exfoliative skin conditions, are pregnant or breastfeeding, or have a concurrent disease or other comorbidities. Decisions about treatment should always be discussed with your medical provider. Treatment can also be used when lesions develop in the eyes, mouth, or other anatomical areas where Mpox virus infection might constitute a special hazard (e.g., the genitals or anus).
- Currently, there is no treatment explicitly approved for Mpox virus infections. However, antivirals developed for use in patients with smallpox may prove beneficial against Mpox. TPOXX is an antiviral medication being used as an investigational treatment. If your medical provider feels this would benefit you, please contact ADH at 1-800-803-7847.
There are vaccines available for people who meet the criteria. It is recommended that you talk with your healthcare provider before being vaccinated. The preferred vaccine to protect against Mpox is Jynneos, which is a two-dose vaccine. It takes 14 days after getting the second dose of Jynneos for its immune protection to reach its maximum. The vaccine is not effective once symptoms have started.

Vaccine Location Map
For Providers
Testing is currently available through several large commercial laboratories, including LabCorp, Quest, Aegis, Sonic Healthcare, and Mayo Labs. For providers planning to test through a commercial lab, there is no need to contact ADH prior to testing. For providers needing to test through ADH’s Glen F. Baker Public Health Lab, call ADH’s Outbreak Response Section first at 501-537-8969.
Specimen receiving is M-F 8am to 7:30pm at PHL. Specimens can be stored at 2-8 C until ready to ship.
Resources:
- CDC
- School FAQs
- Mpox: Get the Facts
- Social Gatherings, Safer Sex and Mpox
- Health Alert Network Advisory: Accessing Tecovirimat (TPOXX) for Patients with Mpox
Pregnancy & Parenting Resources
We can help you find helpful resources for your pregnancy and parenting.
- Call 855-275-6667 (ARK MOMS)
- Or find the information you need by clicking here | En Español
If you need maternity services or assistance with food for you and your baby and children, ADH local health units can assist you in applying for maternity, WIC, and other services.
There is a health unit in each county. A list of all the health units can be found here. Find the one closest to you and make an appointment to get started with your pregnancy care.
Other helpful resources for a healthy pregnancy and parenting tools:
- Arkansas Department of Human Services
- Arkansas Department of Human Services page to apply for health care benefits, Supplemental Nutrition Assistance program (SNAP), and Transitional Employment Assistance (TEA)
- Arkansas Safe Haven
- ADH Maternity Program
- ADH Maternal and Child Health
- COVID-19 & Pregnancy
- Home Visiting Resources
- Be Well Baby Tobacco Cessation
- WIC (Supplemental foods for Women, Infants, and Children)
- Workforce Services
- Positive Parenting Program
Information on Housing
Information on Birth Defects
- Arkansas Birth Defects Program
- Arkansas Center for Birth Defects Research and Prevention
- Arkansas Children's Hospital - Spina Bifida
- Birth Defects in Arkansas
- United Cerebral Palsy of Arkansas
Arkansas Violent Death Reporting System
What is the Arkansas Violent Death Reporting System?
Violent death is a major public health issue in Arkansas. The Centers for Disease Control and Prevention’s (CDC) National Violent Death Reporting System (NVDRS) defines violent death as a death that “results from the intentional use of physical force or power, threatened or actual, against oneself, another person, or a group or community.” The Arkansas Violent Death Reporting System (ARVDRS) collects and links key details of violent deaths- the “who, when, where, and how” – and delivers insights into “why” these deaths transpired.
Arkansas received grant funding from the CDC in 2018 to create the ARVDRS. ARVDRS pulls data from law enforcement reports, death certificates, and coroner/medical examiner reports (including toxicology) and logs this data into a de-identified database. These sources individually provide data that explain violence in a fragmented, narrow context, but when linked together, a more comprehensive picture of violent death is created. The purpose of utilizing the ARVDRS is to implement a plan to collect and disseminate accurate, timely, and comprehensive surveillance data on all violent deaths occurring within the state to better guide prevention efforts.
ARVDRS includes deaths that result from:
- Homicides
- Suicides
- Deaths of undetermined intent
- Unintentional firearm injury deaths
- Deaths due to legal intervention
Resources:
- National Violent Death Reporting System
- NVDRS and Law Enforcement
- NVDRS and Coroner/Medical Examiner Partners
- NVDRS and Vital Statistics Registrars
- Veto Violence
SUDORS (State Unintentional Death Reporting System)
The State Unintentional Drug Overdose Surveillance (SUDORS) is a supplemental program to the NVDRS which is funded through the Centers for Disease Control and Prevention’s (CDC) Overdose Data to Action grant. SUDORS collects detailed information on all fatal overdose deaths in Arkansas.
SUDORS increases the timeliness and comprehensiveness of reporting fatal drug overdoses by capturing information from death certificates, coroner and medical examiner reports, and toxicology reports. These sources include details such as route of administration and risk factors that may be associated with a fatal overdose. The data collected can be analyzed to provide stakeholders with information on overdose prevention response efforts.
Other Resources:
- SAMHSA’s National Helpline: A free, confidential, 24-hour-a-day, 365-a-year, referral helpline. This service provides referrals to local-level treatment facilities, support groups, and community-based organizations. 1-800-662-HELP (4357) or TTY: 1-800-487-4889.
- Start Your Recovery: A free treatment support service locator for anyone dealing with substance use issues that is user-friendly and in a language that’s easy to understand.
- Department of Human Services: Grant-funded treatment facilities in each region of the state.
- NARCANsas app: Available for download on Android and iOS.
- CDC Opioid Overdose Information
| Office | Address | Phone | Fax |
| Substance Misuse & Injury Prevention | 4815 West Markham Street, Slot 10 Little Rock, AR 72205 |
501-683-0707 | 501-682-0427 |
Substance Misuse Education and Prevention
What is substance misuse?
Substance misuse is the use of illegal drugs, alcohol, prescription, or over-the-counter medication in a way that could be harmful or in a way that they are not intended to be used. A substance can be defined as anything that you put into your body that alters how your brain functions. The misuse of substances regularly can alter the way the brain functions. Commonly misused substances include:
- Alcohol
- Opioids (Heroin, oxycodone, fentanyl, hydrocodone)
- Benzodiazepines (diazepam, lorazepam, alprazolam, and clonazepam)
- Over-the-counter cold or cough medicines
- Marijuana (synthetic marijuana)
- Illicit substances (Amphetamines, methamphetamine, cocaine, LSD, ecstasy, and other stimulants)
Substance Misuse Education and Prevention
Substance misuse is a serious public health issue in Arkansas. According to provisional data from the Arkansas Department of Health (ADH) Vital Statistics, in 2021, there were 628 drug overdose deaths in Arkansas. According to the Centers for Disease Control and Prevention (CDC), nearly 841,000 people have died in the United States since 1999 from a drug overdose. The Substance Misuse Education and Prevention Section at the Arkansas Department of Health was established in the fall of 2017 to help address the opioid epidemic and substance misuse. This section is funded through grants from the Centers for Disease Control and Prevention (CDC) and the Substance Abuse and Mental Health Services Administration (SAMHSA).
Reports
Substance Misuse Data Dashboards
2023
2022:
Education and Prevention Programs
- Dose of Reality | PDF
- Peer Recovery Support Specialists
- Naloxone Training/Distribution
- Healthcare Provider Education
Resources
- Centers for Disease Control and Prevention Drug Overdose
- Substance Abuse and Mental Health Services Administration (SAMHSA)
- Arkansas Poison Center
- AR Rx Take Back
- StartYourRecovery.org
- Substance Abuse Prevention - Arkansas Department of Human Services
- National Rehab Hotline
- Addictions.com
- A Free Addiction Resource to Help Those Suffering with Addiction
- Find Free and Affordable Rehab Centers
- Get Clean. Get Sober. Get Detox
| Office | Address | Phone | Fax |
| Substance Misuse & Injury Prevention | 4815 West Markham Street, Slot 10 Little Rock, AR 72205 |
501-683-0707 | 501-682-0427 |
Substance Misuse and Injury Prevention
In the fall of 2017, the Arkansas Department of Health engaged in a small reorganization to build capacity to better address the opioid epidemic. A new branch, Substance Misuse and Injury Prevention was created within the Center for Health Protection. The branch united two existing sections, Injury and Violence Prevention (IVP) and the Prescription Drug Monitoring Program (PDMP).
Programs
- Injury and Violence Prevention
- Substance Misuse Education and Prevention
- Prescription Drug Monitoring Program
- Accidental Poisoning
| Office | Address | Phone | Fax |
| Substance Misuse & Injury Prevention | 4815 West Markham Street, Slot 10 Little Rock, AR 72205 |
501-671-1449 | 501-682-0427 |
COVID-19 and Pregnancy
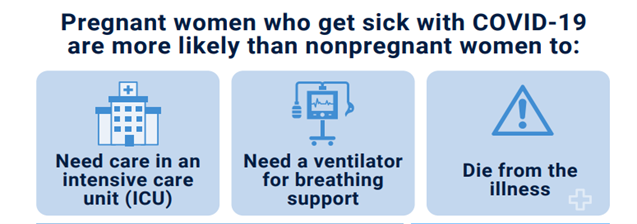
Reports and research show that pregnant and recently pregnant women have a higher risk of more severe illness from COVID-19 than nonpregnant women.
The best way to protect yourself and your baby against COVID-19 is to get vaccinated.
What is COVID-19?
COVID-19 is an illness that affects the lungs and breathing. It is caused by a new coronavirus. Symptoms include fever, cough, sore throat, and trouble breathing. COVID-19 may also cause stomach problems, such as nausea and diarrhea, and a loss of your sense of smell or taste. Symptoms may appear 2 to 14 days after you are exposed to the virus. Some people with COVID-19 may have no symptoms or only mild symptoms.
How does COVID-19 affect pregnant and recently pregnant women?
Reports and research show that pregnant and recently pregnant women have a higher risk of more severe illness from COVID-19 than nonpregnant women. Reports note that:
- Pregnant women who have COVID-19 and show symptoms are more likely than nonpregnant women with COVID-19 and symptoms to need care in an intensive care unit (ICU), to need a ventilator (for breathing support), or to die from the illness. Still, the overall risk of severe illness and death for pregnant women is low.
- Pregnant and recently pregnant women with some health conditions, such as obesity and gestational diabetes, may have an even higher risk of severe illness, similar to nonpregnant women with these conditions.
- Pregnant women who are Black or Hispanic have a higher rate of illness and death from COVID-19 than other pregnant women, but not because of biology. Black and Hispanic women are more likely to face social, health, and economic inequities that put them at greater risk of illness. Visit the Centers for Disease Control and Prevention (CDC) website to learn more about these inequities.
Reports also note the following for pregnant women with COVID-19:
- They may be more likely to have pregnancy complications than pregnant women without COVID-19. These complications may be related to high blood pressure, heavy postpartum bleeding, and other infections. This is especially true for pregnant women with moderate and severe illnesses.
- They may have an increased risk of preeclampsia (a serious blood pressure disorder) and coagulopathy (a blood clotting disorder).
How can COVID-19 affect a fetus or newborn?
Reports of COVID-19 infection during pregnancy have shown the following:
- There is an increased risk of preterm birth.
- Some data suggest a possible increased risk of stillbirth.
- COVID-19 may pass to the fetus during pregnancy, but this seems to be rare.
After birth, reports have shown the following:
- In babies born to women who had COVID-19 during pregnancy, there is an increased risk that the newborn will need care in a neonatal intensive care unit (NICU).
- A newborn can get the virus if they are exposed to it.
Should I get the COVID-19 vaccine?
Yes, you should get a COVID-19 vaccine. The American College of Obstetricians and Gynecologist (ACOG) strongly recommends vaccination if you are pregnant, breastfeeding, or planning to get pregnant. Read COVID-19 Vaccines: Answers From Ob-Gyns to learn more, and talk with your obstetrician-gynecologist (ob-gyn) if you have questions.
Key points to remember:
- Pregnant and postpartum women have a higher risk for more severe illness from COVID-19 than nonpregnant women.
- Stay healthy by getting a COVID-19 vaccine, following guidelines from health officials, and keeping your prenatal and postpartum care visits.
- If you are pregnant and have COVID-19, talk with your ob-gyn.
- Get a booster shot as soon as you are eligible to get one.
Resources for the Community
- Frequently asked questions and answers to COVID-19 Vaccine and Pregnancy
- COVID-19, Pregnancy, Childbirth, and Breastfeeding: Answers from OB-GYNS
- COVID-19, Gynecologist Visit, and Telehealth: Answers from OB-GYNS
- Pregnant and Recently Pregnant Women At Increased Risk for Severe Illness from COVID-19
- Possible Side Effects After Getting a COVID-19 Vaccine
- Myths and Facts about the COVID-19 Vaccine
- Toolkit for Pregnant Women and New Parents
- Keep Your Baby Healthy and Safe Take These Steps During the COVID-19 Pandemic
- Pregnant or Just Had a Baby? Take These Steps to Protect Yourself from COVID-19
Resources for Providers
- Vaccine Confidence Training
- CDC Publications
- COVID-19 Data Tracker: Pregnancy Data-CDC
- Talking with Patients about the COVID-19 Vaccination
Project W at the Arkansas Department of Health
The Arkansas Department of Health is partnering with the Centers for Disease Control and Prevention (CDC) to help protect mothers and babies from the consequences of public health emergencies.
Our health department, in collaboration with CDC, is monitoring pregnant women who test positive for SARS-CoV-2 through the end of their pregnancy and monitoring their infants through at least 6 months of age. We report cases of COVID-19 to CDC as part of CDC’s Surveillance for Emerging Threats to Mothers and Babies Network (SET-NET) (https://www.cdc.gov/ncbddd/aboutus/pregnancy/emerging-threats.html). Data collected as part of these efforts can help direct public health action and inform clinical guidance for the care of affected pregnant women and their infants. You can find the latest data on CDC’s webpage on Birth and Infant Outcomes.
Please contact us at 501-280-4830 or email ADH.ProjectW@arkansas.gov with any questions.
Medicaid Provider Appeals
Medicaid providers may request a fair hearing on any decision or action by the Department of Human Services or its reviewers or contractors that adversely affects a Medicaid provider or client regarding receipt of and payment for Medicaid claims and services including but not limited to decisions as to:
- Appropriate level of care or coding,
- Medical necessity,
- Prior authorization,
- Concurrent reviews,
- Retrospective reviews,
- Least restrictive setting
- Desk audits,
- Field audits and onsite audits, and
- Inspections
Medicaid Provider Fair Hearing requests must be sent to the Arkansas Department of Health, Medicaid Provider Appeals, within 30 calendar days of the date on the notice of adverse action.
Medicaid Provider Appeals may be submitted by U.S. Mail or in-person delivery, by facsimile, or e-mail.
By mail or in-person:
Medicaid Provider Appeals
Arkansas Department of Health
4815 West Markham Street – Slot 31
Little Rock, AR 72205
By fax: 501-661-2357
By email: Jessica.upchurch@arkansas.gov
Arkansas Alzheimer’s Disease & Related Dementias (AADRD)
Defining Alzheimer's Disease and Dementia
What are Alzheimer’s Disease and Related Dementias?
Alzheimer’s Disease
- Alzheimer’s disease involves parts of the brain that control thought, memory, and language. It is a progressive disease beginning with mild memory loss and possibly leading to loss of the ability to carry on a conversation and respond to the environment.
- Alzheimer’s is a specific disease. Dementia is not.
- Alzheimer's disease is the most common cause of dementia, accounting for 60-80% of dementia cases.
- Though the greatest known risk factor for Alzheimer’s is increasing age, the disease is not a normal part of aging.
- Although most people with Alzheimer’s are 65 and older, approximately 200,000 Americans under 65 are living with younger-onset Alzheimer’s disease.
Related Dementias
- Dementia is a general term for a decline in mental ability severe enough to interfere with daily life.
- Dementia describes a group of symptoms associated with a decline in memory, reasoning, or other thinking skill.
- Dementia is not a normal part of aging • Alzheimer's is the most common type of dementia, but there are many kinds:
- Creutzfeldt-Jakob Disease
- Lewy Body Dementia
- Down Syndrome and Alzheimer's Disease
- Frontotemporal Dementia
- Huntington's Disease
- Mixed Dementia
- Normal Pressure
- Hydrocephalus
- Posterior Cortical Atrophy
- Parkinson's Disease Dementia
- Vascular Dementia
- Korsakoff Syndrome
Alzheimer’s in Arkansas
- One out of three seniors who die annually is diagnosed with Alzheimer’s or another dementia.
- Alzheimer’s disease was the 4th leading cause of death among Arkansans aged 65 years and older compared to the national rank as the 5th leading cause of death in 2018.
- Arkansas ranked 23rd among states for Alzheimer’s disease mortality in 2013, however, moved up to 13th in 2018, indicating more Arkansans aged 65 years and older are succumbing to Alzheimer’s disease over time.
Take Brain Health to Heart
What’s good for your heart and blood vessels is also good for your brain. Recent studies have shown that risk factors for heart disease and stroke, such as physical inactivity and obesity, also contribute to conditions such as memory loss, Alzheimer’s disease, dementia, and reduced cognitive ability. Older adults with Alzheimer’s and other dementias are more likely than other older adults to have multiple chronic conditions, and Alzheimer’s complicates the management of these conditions, resulting in increased hospitalizations and costs. The good news is that there are steps you can take that will help your heart and your brain.
10 Ways to Help Improve Your Brain Health
- Don’t Use Tobacco
- Be Physically Active
- Eat a Healthy Diet
- Maintain a Healthy Weight
- Get Enough Sleep
- Stay Engaged
- Prevent and Manage High Blood Pressure
- Prevent and Manage High Cholesterol
- Manage Blood Sugar
- If You Drink, Do So in Moderation
Although age, genetics, and family history cannot be changed, other risk factors can be changed or modified to reduce the risk of cognitive decline and dementia. In fact, the 2020 recommendations of The Lancet Commission on Dementia prevention, intervention and care suggest that addressing modifiable risk factors might prevent or delay up to 40% of dementia cases
The Importance of Caregivers
- About one in three Alzheimer’s caregivers report their health has gotten worse due to care responsibilities.
- Increased risk of stress, depression, unhealthy behaviors, and poor attention to their own health.
- Caregivers of people with dementia with diabetes or osteoporosis were 2.6 and 2.3 times more likely, respectively, to report emotional difficulties with care compared with caregivers of people with dementia who did not have these co-occurring conditions.
What are we doing at the Arkansas Department of Health?
The Arkansas Department of Health is an awarded recipient of the CDC Building Our Largest Dementia (BOLD) Infrastructure for Alzheimer’s Act, Public Law 115-406, for Public Health Programs to Address Alzheimer’s Disease and Related Dementias (CDC-RFA-20-2004). BOLD funding is being used to:
- Increase the number of stakeholders engaged in state-wide AADRD collaboration.
- Promote education about the importance of risk reduction (primary prevention), early diagnosis (secondary prevention), prevention and management of comorbidities, avoidable hospitalizations (tertiary prevention), and the role of the caregiver.
- Increase healthcare professional education about the importance of all three levels of prevention, and the importance of caregivers as a vital part of the healthcare team.
- Increase the number and types of health communications with the integration of brain health and dementia risk.
Rules
Resources:
- What are Alzheimer’s Disease and Related Dementias?
- Providers in Arkansas
- Community Resource Manual
- Alzheimer’s Association
- CDC-Healthy Brain Resource Center
- Arkansas State Plan 2021-2025
- AARP-Family Caring
- UAMS- Center on Aging
- Alzheimer’s Arkansas
Contact:
Jarriel Moore, BA, CBT, CHC
BOLD Program Manager
501-682-6990
Coordinated School Health
**The Coordinated School Health program received funding from the CDC titled (CDC)RFA-DP-23-0002 School-Based Interventions to Promote Equity and Improve Health, Academic Achievement, and Well-Being of Students. The Division of Elementary and Secondary Education, in partnership with the Arkansas Department of Health, are committed to bringing you school health content experts to implement health strategies that benefit students, school staff, and the community. We will continue to host quarterly CSH meetings with topics surrounding the WSCC Model and share relevant resources and funding opportunities as they arise.
Our second quarterly meeting of the 2023-24 school year will be held virtually on Wednesday, November 1, 2023, at 8:30am. You can register in advance for this meeting here. After registering, you will receive a confirmation email containing information about joining the meeting.
Arkansas has emerged as a leader in Coordinated School Health programs. Across the state, Coordinated School Health is growing, and districts are building school-level health teams. With the help of ACT 1220 of 2003, district and school wellness committees are required to assess themselves using the Centers for Disease Control and Prevention's School Health Index, completing modules 1-4, 10, and 11, and then use the results to create an improvement plan. The School Health Index results are used to develop their School Health and Wellness Improvement Plan, which is required for accreditation.
Through grant funding from the CDC, free quarterly professional development opportunities are provided for all school personnel based around the Whole School, Whole Community, Whole Child (WSCC-pronounced “whisk”) Model.
Coordinated School Health Schools
The 132 districts that participate in Coordinated School Health are innovators in their districts and apply for grants and develop plans to improve school health using the scorecard from their School Health Index. Typically, these schools have a high-functioning wellness committee and a tenacious wellness committee chairperson who attends quarterly CSH meetings regularly. If you work for a district and are ready and willing to implement healthy changes and would like to join us, please email the CSH coordinators to be added to the CSH distribution list. You will receive grant notifications, meeting alerts, webinar alerts, quarterly newsletters, and access to all our partners’ updates and alerts as well.
Arkansas Department of Health, CSH Advisor, Ariel Rogers, (501) 280-4148.
Division of Education and Secondary Education, CSH Advisor, Lisa Mundy, (501) 683-3604.
Visit the Arkansas Coordinated School Health webpage to view previous CSH meeting recordings and resources.
You can review data regarding the youth Risk Behavior Survey (YRBS )for Arkansas students by clicking here, and School Health Profiles for Arkansas students by clicking here.
Funding Opportunities
Joint Use Agreements (JUA)
The Arkansas Joint Use Agreement (JUA) Grant is a competitive application process made possible and supported by Arkansas’ governor and the Arkansas Tobacco Excise Tax. These funds help schools adopt and implement joint use policy which allows schools to form community partnerships to maximize resources while increasing opportunities for physical activity. Funds are available each fiscal year based on Tobacco Excise Tax appropriations or until funds are expended. Click here for more information.
School-Based Health Centers (SBHC)
School-Based Health Centers are a great way to coordinate campus services to cover the medical and mental health services students need. Each center must provide basic physical and mental health services no matter their ability to pay. Some centers also include dental services on campus or provide campus-linked services to a provider not located on school grounds. This program functions as a collaboration between the Arkansas Department of Health and the Arkansas Department of Education. The School-Based Health Center funding is a once-a-year competitive grant process where districts can apply for up to $500,000 for a 3-year grant cycle. Former grant cycles lasted 5 years. Click here for more information on School-Based Health Centers.
Student Wellness Advocacy Group (SWAG)
SWAGs are aimed at helping students in 7 - 12th grades learn to advocate for themselves and for healthier campuses. Each year the Arkansas Department of Health funds up to 12 campuses to host SWAGs, providing them with funding, presentations on varying health and advocacy topics, and guidance on advocacy projects in their school, community, or state. For more information about SWAGs click here.
Project Prevent Youth Coalition (PPYC)
Project Prevent is the statewide youth tobacco prevention coalition in Arkansas. For more information on PPYC click here.
Assessment Tools, Data, Resources
State School Health and Wellness
Act 1220 of 2003 created the role of the Act 1220 Coordinator/State School Health and Wellness Coordinator. The Act 1220 Coordinator (State School Health and Wellness Coordinator) works with the 1,102 schools in the 262 districts to ensure the requirements of Act 1220 of 2003 and related state and federal school health mandates are met. The main goal is to lower obesity rates by supporting best practices in food service, nutrition, physical, and health education.
The State School Health and Wellness Coordinator work with school and district wellness committees to help ensure their annual School Health Index (SHI) and School Health and Wellness Improvement Plan are accurately completed and submitted by the October 1st deadline. Also, the Coordinator helps schools in reviewing and updating their School Wellness Policy, provides technical assistance, trainings related to school wellness, and regular updates on school wellness data and reporting.
While the school environment is the primary focus of the State School Health and Wellness Coordinator, the role also works to increase partnerships between communities and their schools. The State School Health and Wellness Coordinator discusses the impacts of student health on academics, long-term health, and community strength. Additionally, communities are encouraged to reach out to local schools to engage in conversations about local school wellness programs and initiatives and how they can support school wellness.
In efforts to increase student driven initiatives in school health and wellness programs, the State School Health and Wellness Coordinator at the Arkansas Department of Health started the Student Wellness Advocacy Group (SWAG) Initiative beginning in the 2018-2019 school year. This program is a partnership between the Tobacco Prevention and Cessation Program (TPCP) and the Office of School Health Servicestoprovide health education and teach students how to have conversations about their health and how to improve it. Advisors work with SWAG participants to discuss content, projects, and how to use funding.
Arkansas Department of Health, State School Health & Wellness Coordinator: Shanetta Agnew, (501) 280-4889.
Division of Education and Secondary Education, State School Health and Wellness Coordinator: Shy Whitley, (501) 683-3604
Wellness Committee Resources
- American School Health Association(ASHA)
- Arkansas Aware
- Arkansas Coalition for Obesity Prevention: Early Childhood and Schools Team
- Be Well Arkansas
- Body Mass Index (BMI) Assessment Data
- BMI Calculator for Child & Teen
- Body Mass Index Dashboard
- BMI Toolkit
- Body Mass Index: A Training Manual
- CDC: Division of Adolescent and School Health (DASH)
- CDC Healthy Schools
- CDC: Virtual Healthy Schools
- Child Health Advisory Committee
- Local School Wellness Policy
- Local School Wellness Policy Implementation Under the HHFK Act of 2010:Summary of Final Rule
- Maximum Portion Size List
- Rules Governing Nutrition & Physical Activity Standards & BMI for Age Assessment Protocols in Arkansas Public Schools
- Wellness Committee Requirements Checklist
- Wellness is Worth It! (Wellness Committee Guide)
- School Health Index
- Smart Snack Calculator
- Society of Health and Physical Educators (SHAPE)
- Student Health Report
- Special Olympics Unified Champion Schools®
- USDA Local School Wellness Policy
Assessment Tools for Wellness Committees
- Comprehensive School Physical Activity Program (CSPAP)
- Health Education Curriculum Analysis Tool (HECAT)
- Physical Education Curriculum Analysis Tool (PECAT)
- School Health Profiles
- WellSAT
- Youth Risk Behavior Survey (YRBS)
Family Resources
- Amaze
- Amaze Jr.
- Arkansas Coalition for Obesity Prevention: Growing Healthy Kids
- CDC: About Child & Teen BMI
- Healthy Family Refrigerator Curriculum
School Nutrition Resources
Additional Data Sources
- Adolescent Behaviors and Experiences Survey (ABES)
- America’s Health Rankings
- Recess Pilot Impact Report
- School Health Policies and Practices Study (SHPPS)
- School Nurse Survey Report
- State of Childhood Obesity
- Act 101 of 2023
- Act 128 of 2023
- Act 286 of 2023
- Act 290 of 2023
- Act 737 of 2023
- Act 1220 of 2003
- Act 201 of 2007
- Act 317 of 2007
- Act 719 of 2007
- Act 1079 of 2015
- Act 1062 of 2017
- Act 641 of 2019
- Act 536 of 2021
- Act 624 of 2021
- Act 767 of 2021
- Act 775 of 2021
- Act 829 of 2021
- Act 886 of 2021
- Act 1002 of 2021
- Act 1070 of 2021
- Act 1074 of 2021
Housing Opportunities for Persons with AIDS (HOPWA) Program
Downloads:
PDMP - Reports and Resources
Reports
| Statewide Evaluation of PDMP Data: Results |
| Statewide Evaluation Conducted 2022-2023 (Dashboard) |
| Statewide Evaluation Conducted 2022-2023 (pdf) |
| Annual Reports: |
| 2022 |
| 2021 |
| 2020 |
| 2019 |
| 2018 |
| 2017 |
| 2016 |
| 2015 |
| County Maps |
| 2022 Opioid Dispensing Rate |
| 2021 Opioid Dispensing Rate |
| 2017 County Map Data |
| 2016 County Map Data |
| 2015 County Map Data |
| 2014 County Map Data |
| 2017 County Tables |
| 2017 Benzodiazepine Data |
| 2017 Opiate Data |
| 2017 Stimulant Data |
| Mortality Reports |
| 2000 - 2016 |
| Other Reports |
| 2020-2022 Prescription and Pill Count Tables |
| Neonatal Abstinence Syndrome |
| Hospitalization Data: 2014 |
Resources
- Academic Detailing:
- The Arkansas Department of Health is partnering with the University of Arkansas for Medical Sciences and the AR-IMPACT program (Improving Multi-disciplinary Pain Care and Treatment) to offer a new service for healthcare providers in the state. Academic Detailing is a nationally supported effort in which a trained pharmacist or physician meet providers one-on-one and discuss, in this case, the most recent national guidelines for safe prescribing of opioids, alternative treatments for chronic pain, and how to taper opioids safely. This service is free of charge and generally takes 10-15 minutes. The academic detailers are available to meet at your place of work before, during, or after regular office hours, and can initially present to larger groups, if requested. The Arkansas Department of Health encourages all providers to utilize this free educational opportunity to help continue to improve patient care in the state. The Academic Detailing team has already begun reaching out to clinics across the state, but if you are interested in this opportunity, please reach out directly to Amanda Lunsford at lunsfordamandak@uams.edu or 501-526-8326.
- AR-IMPACT: Free online CE opportunity for physicians, nurses, and pharmacists via learning on demand.
- AR Takeback
- AR Connect
- CDC Guidelines
- MME Calculator
- DEA Diversion
**Please provide contact information in your email response to our Office.
| Office | Address | Phone | Fax |
|---|---|---|---|
| Prescription Drug Monitoring Program (PDMP) | 4815 W. Markham St. - Slot 10 Little Rock, AR 72205 |
501-683-3960 | 501-369-6794 |
PDMP - For Healthcare Providers
UPDATE TO REPORTING ANIMAL PRESCRIPTION
- Animal Prescription Reporting for Pharmacists
- Animal Prescription Reporting for Vendors
- Animal Prescription Reporting for Veterinarians
- Universal Claim Form for Veterinarians
- Reporting Prescriptions for Animal Patients Tutorial
- FAQs Regarding Reporting Animal Prescriptions
Rules/Laws
Per Act 820 of the 2017 Arkansas legislature, ADH is required to post prescribing criteria to the website. Additionally, various licensing boards were required to promulgate rules limiting the amount of Schedule II narcotics that may be prescribed and dispensed by licensees of each applicable board. The boards required to promulgate such rules include Arkansas State Medical Board, Arkansas State Board of Dental Examiners, Arkansas State Board of Nursing, Arkansas State Board of Optometry, Arkansas State Board of Pharmacy, and the Veterinary Medical Examining Board. The Director of the Department of Health, upon consultation of the Prescription Drug Monitoring Program Advisory Committee, decided to post each boards’ promulgated rules as applicable prescribing criteria for the state. They are linked as follows:
- Dental Practice Act page 89
- Medical Practice Act page 78
- Nurse Practice Act of the State of Arkansas Chapter 4, Section 8.
- Optometry Board page 40
- Veterinarian Board page 16.
- Pharmacy Board
What is the PMP Gateway Integration?
Integrating access to the Arkansas PDMP database within an Electronic Health Record (EHR)/Pharmacy Management System (PMS) provides a streamlined clinical workflow for providers. The integration eliminates the need for providers to have to log in separately to the PDMP. Instead, the EHR/PMS automatically initiates a patient search and returns a view of the patient’s controlled substance prescription history report directly within the provider's EHR/PMS.
PMP Gateway is a multi-state query system that provides access to a majority of state PMPs. Bamboo Health, through its PMP Gateway product, facilitates communication, information transfer, integration, and support for the state approval process, and the EHR/PMS vendor development process.
Dispenser Reporting
As mandated, a dispenser is required to report the dispensation of any controlled substance that is schedule II – V as listed on the Arkansas Pharmacy Controlled Substance List to the Arkansas Department of Health Prescription Drug Monitoring Program. A pharmacy is required to report at a frequency of the next business day while a veterinary clinic is required every 30 days.
If no controlled substances were dispensed, then a zero report is required. If a pharmacy does not dispense controlled substances, the pharmacy may apply for a waiver exemption. Please email a completed Pharmacy Waiver form to the AR PDMP to apply.
- PMP Clearinghouse
- Clearinghouse User Guide
- PMP Clearinghouse Customer Support: 1-855-729-8917
Tutorials and Guides for the PDMP
User Guides & Best Practices
- User Guide for Clearinghouse
- User Guide for PMP AWARE
- Best Practices for Reporting Guide
- Prescriber Comparison Report Guide
**Please provide contact information in your email response to our Office.
| Office | Address | Phone | Fax |
|---|---|---|---|
| Prescription Drug Monitoring Program (PDMP) | 4815 W. Markham St. - Slot 10 Little Rock, AR 72205 |
501-683-3960 | 501-369-6794 |
Full Independent Practice Credentialing Committee
The Full Independent Practice Credentialing Committee was created by Act 412 of 2021. This legislation provides a pathway to full practice authority for Certified Nurse Practitioners (CNP). Legislative changes in 2023 (Act 872) expanded Full Independent Practice to Clinical Nurse Specialists (CNS). The Committee accomplishes this by reviewing the applications submitted by CNPs and CNSs who apply for full independent practice authority.
Pursuant to ACA §17-87-314(e) the responsibilities of the Committee are:
- Review and approve or deny all applications for a certificate of full independent practice authority for CNPs and for renewal of a certificate of full independent practice authority;
- Review complaints made against CNPs and CNSs who have a certificate of full independent practice authority;
- Review recommendations made by the Arkansas State Medical Board and the Arkansas State Board of Nursing and notify the CNP or CNS of any action taken by the Committee based on the recommendations;
- Hold a hearing if the action taken is suspension or revocation of the certificate of full independent practice authority; and
- Provide reports quarterly and upon request regarding the number of applicants approved and denied to the Senate and House Committees on Public Health, Welfare, and Labor.
Members
The Full Independent Practice Credentialing Committee is an eight-member committee created within the Arkansas Department of Health and appointed by the Governor. Committee members serve three-year terms, but not more than two consecutive terms. The committee shall consist of the following members:
- Three (3) faculty physicians from each of the following institutions:
- The College of Medicine of the University of Arkansas for Medical Sciences
- The Arkansas College of Osteopathic Medicine in Fort Smith
- The New York Institute of Technology College of Osteopathic Medicine at Arkansas State University in Jonesboro
- One (1) physician from the state at large
- Three (3) faculty CNPs from nursing programs in the state
- One (1) CNP from the state at large
The Director of the Arkansas State Medical Board and the Director of the Arkansas State Board of Nursing serve as ex officio nonvoting members.
| Name | Position | Appointed |
| Mitzi Scotten, MD | NYIT College of Osteopathic Medicine – Fort Smith | 9/1/21 |
| Donna Shipley, MD | AR College of Osteopathic Medicine – Fort Smith | 9/1/21 |
| William Hawkins, MD | At-Large | 9/23/21 |
| Darlene Byrd, DNP, APRN | UAMS | 9/1/21 |
| Leonie DeClerk, PhD, DNP, APRN | UAMS | 9/1/21 |
| Mark Foster, DNP, APRN Vice Chair |
ASU | 9/1/21 |
| Julia Ponder, DNP, APRN Chair |
At large | 9/1/21 |
Meetings
- July 23, 2024
- October 29, 2024
Minutes
- January 16, 2024
- October 31, 2023
- August 22, 2023
- June 27, 2023
- May 23, 2023
- April 25, 2023
- February 28, 2023
- January 17, 2023
- December 6, 2022
- November 1, 2022
- October 4, 2022
- September 13, 2022
Resources
Act 412 of 2021 allows a pathway for certified nurse practitioners (CNPs) or clinical nurse specialists (CNSs) to apply for full independent practice after having practiced 6,240 hours under a collaborative practice agreement.
Applications shall be submitted through the Arkansas Nurse Portal. Once all required documents have been received, applications will be sent for review by the Full Independent Practice Credentialing Committee (FIPCC). The FIPCC will meet at least quarterly to review applications.
Instructions to Access the Application:
- Sign in to the Arkansas Nurse Portal account.
- Scroll down to the “Other Applications” section.
- Click the “Apply” button.
- Click on Full Practice Authority for CNPs/CNSs
- Complete the application by following the instructions.
- Upload any required documents.
- Two letters of recommendation
- Submit completed and notarized Practice Hours Affidavit (signed by a collaborating physician(s)).
- Evidence of five (5) advanced pharmacology continuing education hours obtained within the last two (2) years.
- Resume or curriculum vitae showing past work history as an APRN.
- Click submit.
- Pay the required fee.
- After submission, ASBN staff will review the application for all required documents. Requests for additional information or documents will be requested through the Arkansas Nurse Portal Message Center.
- The Full Practice Authority Credentialing Committee meets quarterly to review applications. Once the application has been approved or denied, the APRN will receive a letter from the committee through the Nurse Portal Message Center.
- Until approval has been received, the APRN is required to maintain a current collaborative practice agreement.
Contact Us
Full Independent Practice Credentialing Committee
4815 W. Markham St., Slot 75
Little Rock, AR 72205
Email
State Health Assessment Report
OPMQIE | Performance Management System | Quality Improvement Plan | Strategic Plan
State Health Improvement Plan | Workforce Development Plan
The State Health Assessment 2020, Arkansas’s Big Health Problems Report provides scientific evidence of the health status of people in Arkansas. It is the foundation upon which the State Health Improvement Plan and the Agency’s Strategic Plan are based. The report is an updated edition of the 2013 publication “Arkansas’s Big Health Problems and How We Plan to Solve Them”. It is available in print or book versions as well as in PDF format.
The State Health Assessment 2020 Report provides information on the risk factors contributing to poor health outcomes among Arkansans. It also provides public health, health care, and other resources available in the community to help improve the health status of the population.
Access to the full State Health Assessment 2020, as well as specific chapters, can be found here.
- State Health Assessment 2020 (pdf)
- Individual Chapters
- Executive Summary
- Chapter 1: The People of Arkansas and Their Health
- Chapter 2: Life Expectancy
- Chapter 3: Infant Mortality
- Chapter 4: Health Literacy
- Chapter 5: Social and Behavioral Determinants of Health
- Chapter 6: Rural Health
- Chapter 7: Giving Everyone a Chance at Good Health
- Chapter 8: Emerging Public Health Issues
- Chapter 9: Resources for More Information
- Please see the State Health Assessment Scorecard here.
Early Hearing Detection and Intervention Performance
Reports:
- FY24 Annual Report
- FY24 Annual Birthing Hospital Survey Highlight
- 2022-2023 Birthing Hospital Performance Infographic
- 2023 Non-Permanent Loss Survey Analysis
- FY23 Annual Report
- 2023 Annual Report Screening Data
- 2023 Annual Report Diagnostic Data
- 2023 Annual Report Early Intervention Data
- FY23 Annual Birthing Hospital Survey Highlight
- FY22 Annual Report
- FY22 ERAVE Usability Evaluation
- 2019 Infant Hearing Program Infographic
Electronic Case Reporting (eCR)
The Arkansas Department of Health is actively accepting and onboarding facilities for electronic case reporting. Please contact the Office of Health Information Technology at SHAREhealth@arkansas.gov or call at 501.410.1999.
What is Electronic Case Reporting (eCR)?
Electronic case reporting (eCR) is the automated, real-time exchange of case report information between electronic health records (EHRs) and public health agencies. It moves data quickly, securely, and seamlessly from EHRs in healthcare facilities to state and local public health agencies.
eCR uses a centralized platform that allows systems to communicate with each other in real-time. eCR runs behind the scenes in the EHR to automatically capture and report required information. If the information entered in the EHR matches codes of interest to public health, the data necessary for a public health report are sent to the platform. If the data meet jurisdictional reporting requirements, the report is sent to the appropriate public health agencies for investigation and follow-up.
eCR is a joint effort of the Association of Public Health Laboratories, the Council of State and Territorial Epidemiologists, and CDC. These organizations play key roles in leading, implementing, and operating eCR with healthcare organizations, EHR vendors, and public health agencies.
Benefits of Reporting using eCR:
eCR benefits everyone involved in case reporting. It provides timely and more complete data than manual reporting and decreases the burden on both healthcare facilities and public health staff.
For Public Health Agencies:
- Provides timely and complete data to support outbreak management and monitor disease trends
- Efficiently monitors the spread of reportable diseases during outbreaks and public health emergencies
- Reduces response time with automated information
- Improves communication and collaboration with healthcare by enabling bidirectional data exchange
- Supports the submission of case-based data (without identifiable information) to CDC through the National Notifiable Diseases Surveillance System.
For Healthcare Providers:
- Reduces burden for healthcare providers without disrupting the clinical workflow
- Saves time by eliminating manual data entry and reporting
- Can fulfill legal reporting requirements
- Provides real-time reports to public health officials to guide the state, tribal, local, and territorial response to public health threats
- Facilitates communication and collaboration between healthcare and public health
- Streamlines reporting to multiple jurisdictions
- Receives information from public health associated with the reportable condition
- Can be implemented for all reportable conditions
For more information, please click here.
Informacion de Vacunacion - COVID-19
COVID-19 Recursos en Espanol | Pruebas de Deteccion de COVID-19 |
Vacunacion Contra el COVID-19
Aunque el desarrollo de la vacuna contra el COVID-19 ha sido un proceso acelerado, la seguridad ha sido la máxima prioridad.
¿Quién es elegible para recibir la vacuna en Arkansas?
Todos los habitantes de Arkansas de 6 meses o mayores ahora son elegibles para recibir la vacuna.
¿Cómo puedo obtener la vacuna?
Los residentes de Arkansas elegibles pueden programar una cita con un proveedor de vacunas. Las clínicas y eventos de vacunación pueden estar disponibles en su área a través de hospitales, proveedores de atención médica, o en su lugar de trabajo.
Pruebas de Deteccion de COVID-19
COVID-19 Recursos en Espanol | Plan de Vacunacion - COVID-19
Pruebas
Si tienen fiebre, tos, o dificultad para respirar o si cree que ha estado expuesto a COVID-19, llame anticipadamente a su médico o solicite una cita para hacerse la prueba en la Unidad Local de Salud. Las pruebas están disponibles.
Use el mapa que se encuentra abajo para encontrar un lugar que ofrece pruebas cerca de usted. En las Unidades Locales de Salud, las cuales se encuentran marcadas en rojo, las pruebas son gratuitas y no se requiere tener seguro médico. Llame primero para hacer una cita.
Arkansas State Medical Board
Mission
The Medical Board's mission is to protect the public and act as their advocate by effectively regulating the practices of Medical Doctors, Osteopathic Medical Doctors, Physician Assistants, Respiratory Therapists, Occupational Therapists, Occupational Therapy Assistants, Radiology Practitioner Assistants, and Radiologist Assistants, Licensed Genetic Counselors and registration of Surgical Assistants and Medical Corporations.
- Arkansas State Medical Board full website
- Board Members
- Directions to the Board Office
- Board Definitions & Terminology
- History of the Board
- Board Meetings
- Licensing
- Regulatory/Discipline
- Internet Policies & General Information
Announcements
- Act 313 - Required Changes at Healthcare Facilities and Clinics (effective August 1, 2023)
Services
- Medical Corporations Start Renewal Here
- Applicant Portal
- Licensee Login
- Verify a License
- Directory Search
- Online Services Registration
- Order a Board Certification: cost of service is $15; can also mail a written request to the Board Office
- Purchase a Mailing List
- Forms and Publications
For the Practitioner
Continuing Medical Education
- Healthcare-associated infections (HAIs) Training
- UAMS Office of Continuing Education (OCE)
- UAMS Impact
- Optimizing Perioperative Pain Management: An evidence-based approach
Resources
- Act 670
- Naturopathic Study Submission
- License Actions
- Recently Licensed
- Expiring Licenses
- Licensing Statistics
- Frequently Asked Questions
- Outside Resources
| Office | Address | Phone |
| Arkansas State Medical Board | Victory Building 1401 West Capitol, Suite 340 Little Rock, AR |
Office: 501-296-1802 Fax: 501-603-3555 |
Arkansas Spinal Cord Commission

The Arkansas Spinal Cord Commission is a health-related agency of the state of Arkansas, established by Act 311 of 1975 and administered in accordance with Arkansas Code Annotated (ACA ) 20-8-201 – 206. Click here to view the Legislative Mandate Arkansas Code Annotated (ACA).
Our Mission
The Arkansas Spinal Cord Commission will administer a statewide program to identify and meet the unique and lifelong needs of people with spinal cord disabilities in Arkansas.
Our Purpose
To assist Arkansans with spinal cord disabilities in living as independently as they choose.
Our Goals
- Provide effective Case Management services to all eligible clients to improve independence and function.
- Conduct comprehensive consumer-driven service programs to meet client needs.
- Provide education and resources to individuals with spinal cord disabilities, their families, health care professionals, and the general public.
- Research the specialized needs, issues, trends, services, and health care resource utilization of individuals with spinal cord disabilities and make recommendations for change.
- Identify state, federal, and private funding sources to access additional funds for the provision of client services, research, and innovative program development.
- Network with state, federal, public and private agencies to coordinate services and resources and to advocate for the needs of people with spinal cord disabilities.
- Operate the Arkansas Spinal Cord Disability Registry, promoting optimal and timely reporting, surveillance, data collection and analysis, and dissemination of registry information.
- Disseminate the results of ASCC spinal cord disability registry analysis and other research projects to compare with other states, systems, and national data.
- Utilize sound organizational management plan to assure effective, efficient fiscal, personnel, and programmatic services.
- Market and promote the activities and services of the Commission and the successes of our clients.
About Us
- History of the Arkansas Spinal Cord Commission
- Commission Members
- Office and Case Manager Locations | Coverage Map
- Conferences
- What is a Spinal Cord Disability?
Commission Meetings
Five (5) members are appointed by the Governor of Arkansas for terms of ten (10) years. The members of the commission shall be either spinal cord injury victims themselves, members of the immediate families of spinal cord injured victims, or persons with special knowledge of and experience with spinal cord injuries and dysfunctions who have demonstrated active involvement and interest in the fight against death and disability due to spinal cord injury and dysfunction.
Regular meetings are held quarterly in February, May, and August on the last Tuesday of the month and in November on the third Tuesday of the month except when changed by the majority.
Special meetings may be called by the Chairperson or requested by a majority of the members as needed with three business days' notice to members in order to transact only business stated in the notice.
Upcoming Meetings:
- May 28, 2024 at 1pm
Publications & Reports (for any publications older than 2023, contact the Commission office)
Annual Reports: 2023
Newsletters: Fall 2023
Manuals:
Resources:
Office hours: M-F 8am - 4:30pm
| Office | Address | Phone | Fax |
| Arkansas Spinal Cord Commission | Freeway Medical Building - Suite 108 5800 W 10th St. Little Rock, AR 72204 |
501-296-1788 1-800-459-1517 |
501-296-1787 |
OHIT - SHARE
Patient medical information has historically been stored on paper in filing cabinets at various medical offices. In recent years, many clinics and hospitals have shifted from paper charts to electronic health records. More than 37 percent of office-based physicians in Arkansas implemented an EHR system in 2011(according to the Department of Health & Human Services), and the adoption of EHR systems continues to grow. In 2013, all Arkansas hospitals are using an EHR system, and experts estimate that more than 60 percent of office-based Arkansas physicians currently use an EHR system.
A digital health care infrastructure provides many benefits over paper systems. But just like paper charts, electronic health records are stored within a clinic or hospital system and are rarely shared with your other providers. As a result, your doctors seldom have access to all your health information, which they need to give you the best care possible.
The Arkansas State Health Alliance for Records Exchange (SHARE) is a state-wide health information exchange (HIE) that solves this problem. SHARE allows participating doctors, nurses, specialists, health services professionals, and public health authorities to access and securely exchange with each other real-time, electronic patient information that is protected by federal and state privacy and security laws.
SHARE provides health information in a standardized electronic format and enables medical data to follow patients rather than being housed in separate physician’s offices or within a single hospital system. SHARE can greatly improve the completeness of patient’s records, which can have a major effect on care as medical history, current medications, and other information is reviewed during visits.
Appropriate, timely sharing of vital patient information through SHARE can better inform decision-making at the point of care and allow providers to:
- Avoid hospital readmissions
- Avoid medication errors
- Improve diagnoses
- Decrease duplicate testing
- Improve patient care coordination
- Promote improved management of chronic diseases.
Participating SHARE providers have access to their patients’ lab results, X-ray reports, medication allergies, and other vital information. By viewing health histories in SHARE, health care providers will have more complete medical information to provide high-quality care and coordinate treatment with other health care providers.
Click here for OHIT-SHARE full website.
Arkansas Social Work Licensing Board
The Social Work Licensing Board was created by Act 791 of 1981 for the purpose of regulating the practice of social work in Arkansas
The mission of the Social Work Licensing Board is to protect the public by setting standards of qualification, training, and experience for those who seek to represent themselves to the public as social workers and by promoting high standards of professional performance for those engaged in the practice of social work.
Online Services
Laws & Rules
Laws and Rules of the Arkansas Social Work Licensing Board.
- Arkansas Social Work Code/Law
- Arkansas Social Work Rules (effective 01/01/2022)
- Military Member Licensure
*Dates the notice is running October 20-22, 2023
*End of public comment November 20, 2023
The Board meets on the second Monday of each month in The Freeway Medical Building at 5800 West 10th Street, Little Rock, AR. The meetings are open to the public.
Click here for a list of the scheduled board meetings/hearings for 2024.
Please note: Meeting times and dates may be subject to change. Every effort will be made to keep the most current information available.
The deadline for submitting documentation for review at a Board meeting is always Thursday before the meeting. Meetings begin at 10:00 a.m. unless otherwise noted at https://portal.arkansas.gov on the Public Meeting Calendar Page.
- About the Board
- Contact the Board
- Meeting Minutes: For any minutes older than 2022, contact the Board office.
Continuing Education
Social Workers must complete thirty (30) hours of social work continuing education during the two-year license period to be eligible for license renewal.
Social Work Continuing Education (SWCE) has been defined as those formalized activities that are directed at developing and enhancing an individual's social work knowledge base and service delivery skills in the applicable area of planning and administration, education, research, or direct service with individual, couples, families, and groups. These activities may include short academic courses, audit courses in colleges and universities, independent study courses, internet courses, workshops, seminars, conferences, and lectures oriented toward the enhancement of social work practice, values, skills, and knowledge.
The Board does not have the capacity to pre-approve all continuing workshops. It is the licensee's responsibility, using his/her professional judgment, to determine the workshops that are applicable and appropriate to his/her professional development as a social worker. The continuing education guidelines may be found here.
Forms
PDF forms below require the Free Adobe Acrobat Reader to View or Print. Forms that are not available for download or submission on the internet must be obtained by contacting the Board office at swlb@arkansas.gov.
- Application Checklist and Instructions
- Application Checklist (other)
- Application Form
- Verification of Licensure in another state
- Renewal Application Packet
- Form for Reporting Continuing Education
- Supervision Plan
- Supervision Evaluation Form
- LCSW Supervision Guidelines
- Address/Name Change Form
- Replacement License Request
- Certificate of Registration Application
- Certificate of Registration Renewal
Complaints and Disciplinary Actions
The Board is authorized to receive complaints against licensees or applicants from any person. A complaint form may be obtained from the board office or this website. The Board may in its own motion, in the absence of a written complaint, initiate its own complaint and conduct an investigation of a suspected violation if reasonable cause exists to believe a violation has occurred.
Click Here for Licensing Information and FAQs
Other Resources
- Arkansas Social Work Programs Accredited by the Council on Social Work Education
- Social Work-Related
Staff:
Kristen Allen, Director of the Board
Chere' Johnson, Administrative Specialist III
| Office | Address | Phone |
| Arkansas Social Work Licensing Board |
Mailing: Physical: |
Office:(501) 372-5071 Fax: (501) 372-6301 |
Arkansas State Board of Dispensing Opticians
The Board is charged with administering, coordinating, and enforcing the Ophthalmic Dispensing Act codified at Ark. Code Ann. 17-89-101 et seq. "Ophthalmic Dispensing" includes the preparation of laboratory work orders, verification and dispensing of spectacle lenses, spectacles, eyeglasses, or parts thereof to the intended wearer, on written prescription from ophthalmologists or optometrists. More detail is provided in Ark. Code 17-89-102 (1)(A)&(B).
The Board's duties include evaluating qualifications to take the licensing examination, establishing the format and content of the examination, and administering the written and practical examination for licensed dispensing opticians. The Board then issues a certificate of licensure or certificate of registry to those applicants who successfully meet the qualifications and pass the examination.
The Board investigates complaints, allegations, and charges of practices that violate the provisions of the Ophthalmic Dispensing Act and the rules and regulations promulgated pursuant to the Act. These complaints are against licensed dispensing opticians and against a business that is acting as licensed dispensing opticians without being licensed.
The Board compiles and maintains a book of licensure and registry of all Dispensing Opticians who are licensed are registered to engage in the business of ophthalmic dispensing in the State.
The Board promulgates rules which establish which acts shall constitute improper conduct and grounds for revocation or suspension of a license or registry or for refusal to renew a license or registry. It also promulgates rules and regulations conforming to the policies of the Act for the purpose of carrying into effect the purpose of the Act. This includes establishing ethical standards of ophthalmic dispensing practices, application procedures, and procedures for investigating complaints.
The Board registers apprentice dispensing opticians who work with licensed or registered opticians.
The duties and functions of the Board are listed completely in Ark. Code Ann. 17-89-203.
Forms & Publications
- Application for License Exam
- Application: Apprentice Disp. Optician License
- Certificate of Ownership
- Change of Address or Employment
- Supervision Agreement
- Office Permit Application
- Office Permit Renewal Form
- Apprentice Application and Renewal Form
- Letter for Office Permit Renewal Form
- Letter for Optician Renewal
- Apprentice Quarterly Supervision Report-One Supervisor
- Retest Application
- Request for ABO Approved CEU Course Approval and Certificates
Resources:
- Board Members
- Licensed Opticians Directory
- Apprentice Directory
- Rule Updated
- Law
- Notice of Rulemaking
- Opticians Association of Arkansas
- Military Member Licensure
The Arkansas State Board of Dispensing Opticians’ licensed and apprentice certificates must be renewed annually by July 1st. Pursuant to Board Rule 144.00.1-11. Annual Renewal, Licensees must obtain four (4) hours of the required continuing education in even-numbered years (2020).
| Office | Address | Phone | Fax |
| Arkansas State Board of Dispensing Opticians Contact: Jerry Himes, Secretary-Treasurer |
P.O. Box 627 Helena, AR 72342 |
870-572-2847 Cell: 601-954-1278 |
870-572-2847 |
ASBEADAC - Licensure Information
A two-tier licensure system is currently available. The most significant difference in the level of licensure relates to the level of education:
- Licensed Alcoholism and Drug Abuse Counselor (LADAC) – holds a masters degree in the health or behavioral sciences field or another appropriate field from an accredited college or university;
- Licensed Associate Alcoholism and Drug Abuse Counselor (LAADAC) – holds a baccalaureate degree in the health or behavioral sciences field or another appropriate field from an accredited college or university;
All applications are reviewed by the Credentialing Committee and presented to the Board for approval at the next regularly scheduled meeting following receipt of all required documents.
- Licensure Submission Checklist
- Registration Application
- Code of Ethics Signature page
- Code of Ethics
- Act 443 of 2009
- Supervision Verification
- Supervised Work Experience
- Pre-Licensure Criminal Background Check
- AR State Police Criminal Background Check
Relicensure Information
Arkansas State Board of Examiners of Alcoholism and Drug Abuse Counselors
“This Board is authorized by Arkansas Code Annotated section 17-27-401 to 416 to license alcoholism and drug abuse counselors and to regulate such licensees to protect the public from unqualified or unprofessional persons holding themselves out to the public to be licensed alcoholism and drug abuse counselors. The Board is also authorized to investigate complaints and sanction licensed alcoholism and drug abuse counselors who violate the rules or ethics code of the Board.”
Our mission is to protect the public by upholding the standards of practice for alcoholism and drug abuse counselors.
On behalf of the members of the Board and staff, we hope these web pages will prove to be a useful tool for both the public and for certified and licensed alcohol and drug abuse personnel.
For individuals seeking licensure in the State of Arkansas, click here.
About Us
I. Purpose
This Scope of Practice Statement is intended to provide a definition of professional alcoholism and drug abuse counselor’s rights and responsibilities and to distinguish this profession from other behavioral healthcare professionals.
II. Introduction
Alcohol and drug abuse counseling is the application of general counseling theories and treatment methods adapted to specific alcohol and drug theory and research, for the express purpose of treating persons with alcohol and other drug problems and persons with co-occurring psychiatric disorders within our diverse society.
III. Foundations of Alcohol and Drug Counseling
The practice of alcohol and drug counseling is based on the following knowledge:
- Pharmacology and psychopharmacology of alcohol and drugs (drugs of abuse and drugs used in the treatment of addictions and other psychiatric disorders).
- Addiction processes including models and theories of addiction; the social and cultural context of addiction; the biological, psychological, and social effects of addiction; and the differentiation and interrelations of addiction from other medical and psychological conditions.
- Various treatment models and methods including models of treatment, relapse prevention, and continuing care; the impact of treatment on problems associated with addiction; co-occurring psychiatric disorders; the importance of community, social, family and self-help systems.
- Practical application including use of interdisciplinary approaches and teams in treatment; assessment and diagnostic criteria; appropriate use of treatment modalities; adapting treatment strategies to a patient’s individual characteristics and needs; and the use of other resources in securing the best available services for the patient.
- Professional standards of practice including recognizing the needs of diverse populations relating to issues of ethnicity, race, gender, sexual orientation, HIV/AIDS, and co-occurring psychiatric disorders; adherence to ethical and professional standards of conduct; commitment to continuing education and clinical supervision; awareness of policies and procedures for patients and staff safety; an understanding of etiology, treatment and prevention; and the clinical application of current research in alcohol and drug treatment.
IV. Scope of Practice
The practice of alcohol and drug counseling consists of the activities listed below. The practice of these activities will conform to the individual’s level of training, education, and supervised experience.
1. Clinical Evaluation of Drug and Alcohol Issues
1.1 Screening of Alcohol and Drug Problems
1.2 Assessment of Alcohol and Drug Problems
1.3 Screening for the presence of other Psychiatric Disorders
1.4 Diagnosis of Alcohol and Drug Problems
2. Treatment Planning
2.1 Case Management
2.1.1 Implementing the Treatment Plan
2.1.2 Consulting
2.1.3 Continuing Assessment and Treatment Planning
2.2 Referral
2.3 Cultural Diversity
2.4 Patient Advocacy
3. Counseling
3.1 Individual Counseling
3.2 Group Counseling
3.3 Family Counseling
4. Education and Prevention
4.1 Patient
4.2 Family
4.3 Community
5. Documentation
6. Professional and Ethical Standards
Board Meetings:
Resources:
- Board Members & Contact Information
- Rules
- Standards of Practice
- Scope of Practice
- Code of Ethics
- Military Member Licensure
| Office | Address | Phone | |
| Arkansas State Board of Examiners of Alcoholism and Drug Abuse Counselors |
4815 West Markham |
Phone: 501-614-5293 |
ARBEADAC@Arkansas.Gov |
Arkansas State Board of Examiners in Counseling
Welcome To The Arkansas Board of Examiners in Counseling and Marriage & Family Therapy.
The Board of Examiners is here to help you pursue a professional license to practice in Arkansas. Our web pages provide access to application forms, licensure protocol, laws, rules, disciplinary procedures, rosters of practitioners and supervisors, and other helpful information that will assist you in your chosen career.
The Board also invites comments and questions from consumers. If you wish to locate a counselor, or if you have questions or concerns about the professionals who serve the citizens of our state, you can find it on this website.
We hope you will visit our website often to stay current on the latest developments pertaining to your pursuits. Feel free to contact us with any ideas or suggestions for improving our service.
To read more, visit the BOARD INFORMATION section.
Applicants | Licensees | License Search & Verification | Complaints
Important Information
Laws, Rules, and Codes of Ethics
The enabling legislation of the Arkansas Board of Examiners in Counseling is codified in Arkansas Code Annotated §17-27—101 et seq. and it provides for the licensure and regulation of Counselors and Marriage and Family Therapists; and the individuals who are allowed to use the titles “licensed professional counselor”, “licensed associate counselor”, “licensed marriage and family therapist”, and “licensed associate marriage and family therapist”. The Act is both title and practice.
The Board of Examiners in Counseling shall, in all deliberations and in all adopted Rules, diligently pursue goals most consistent with the public interest and protection of the public welfare and shall, at all times, apply the provisions of Arkansas Code Annotated § 17-27-101, et seq. and the Rules in a fair and impartial manner.
- Rules
- 2014 ACA Code of Ethics
- 2015 AAMFT Code of Ethics
- Military Member Licensure
- Out-of-State Licensure
Document Library
- 6 Month Evaluation
- CE Requirements
- LAC-LPC Core Curriculum Summary
- LAMFT-LMFT Core Curriculum Summary
- License Change Petition
- License Verification
- NCMHCE Petition
- Reference Letter
- Statement of Intent
- Supervision Agreement
- Supervision Hours Reporting Form
- Supervision Termination Notice
Thank you for your interest in Arkansas licensure. Please click the link below to establish access to the applicant portal if you are interested in submitting an application for any of the below:
- Licensed Associate Counselor
- Licensed Associate Marriage & Family Therapist
- Licensed Professional Counselor
- Licensed Marriage & Family Therapist
- Board Approved Supervisor Status
- LAC to LPC License Change
- LAMFT to LMFT License Change
Click here for the Applicant Portal.
Please click here to access your online license profile.
Board Information
Board's History and Organization
2024 Upcoming Meetings: All board meetings are open to the public.
- August 2-3
- August 13 (Hot Springs, Arkansas Behavioral Health Institute-Hot Springs Convention Center)
- September 6-7
- October 4-5
- November 1-2
- November 13 (Little Rock, Arkansas Counseling Association Conference-Wyndham Riverfront Hotel)
- December 6-7
Board Members
- Dr. Suzanne Casey, MFT Practitioner
- Dr. Robbie Cline, LPC Practitioner
- Sherry Holliman, Public Representative
- Dr. Larry Hopkins, Counselor Educator
- Dr. Ryan Martin, Counselor Educator
- Dr. Justin Moore, Counselor Educator
- Anderson Neal, 60+ Representative
- Daniel Sheaffer, LPC/LMFT Practitioner
- Christopher Skrivanos, LPC Practitioner
Meeting Minutes (For any meeting minutes older than 2023, contact the Board's office)
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
| Office | Address | Phone | |
| Arkansas State Board of Examiners in Counseling |
5800 W. 10th St. - Suite 405 |
501-683-5800 | arboec@arkansas.gov |
ASBCE - Procurers
ACT 513 of 2013 went into effect on Friday, 8/16/2013.
17-81-107. Use of a procurer.
(a) As used in this section:
(1) (A) "Procurer" means a person or entity who for pecuniary benefit procures or attempts to procure a client, patient, or customer by directly contacting the client, patient, or customer in person, by telephone, or by electronic means at the direction of, request of, employment of, or in cooperation with a chiropractic physician.
(B) "Procurer" does not include a provider or a person that procures or attempts to procure a client, patient, or customer for a provider through public media or a person that refers a client, patient, or customer to a provider as otherwise authorized by law; and
(2) "Public media" means telephone directories, professional directories, newspapers and other periodicals, radio and television, billboards, and mailed or electronically transmitted written or visual communications that do not involve in-person or direct contact with specific prospective clients, patients, or customers.
(b) A chiropractic physician who uses a procurer is required to:
(1) Have a written contract with the procurer or procurement company with whom the chiropractic physician engages; and
(2) Register the name of any procurer with whom the chiropractic physician contracts with the Arkansas State Board of Chiropractic Examiners. [Acts 2013, No. 513, § 3].
17-81-108. Rulemaking and enforcement. -The Arkansas State Board of Chiropractic Examiners shall establish rules to enforce the requirements of this chapter. [Acts 2013, No. 513, § 3].
D. ADVERTISING BY CHIROPRACTIC PHYSICIANS.
9. Telephone communication including advertising/marketing. Any agent, procurer, contractor, or employee communicating with a prospective patient on behalf of a chiropractic physician shall disclose how the agent, procurer, contractor, or employee obtained the prospective patient's information. The agent, procurer, contractor, or employee must communicate his or her legal name and the name of the particular chiropractic physician on whose behalf the communication is being made, that he or she is an agent, procurer, contractor, or employee of the particular chiropractic physician. Unless such communication of the agent, procurer, contractor, or employee is true and evidence of the same is on file with the Board, the agent, procurer, contractor, or employee shall not state that he or she practices or is employed as attorney, insurance adjuster, chiropractor, and is not employed in the fields of law, health care, law enforcement, private investigation, or insurance.
(a) When direct in-person solicitation is made by an agent, procurer, contractor, or employee of the chiropractor, in addition to the requirements set forth in paragraph 9, the agent, procurer, contractor, or employee shall show the person being solicited a photo ID with their legal name and the name of the chiropractic physician on whose behalf the solicitation is being made, and shall dispense a professional card bearing his or her legal name, and the name, address, and telephone number of the licensed chiropractic physician on whose behalf the solicitation is being made. Such professional card shall be provided to the person being solicited at the beginning of the encounter, and shall be left with the person regardless of whether the person being solicited accepts the solicitation request.
(b) The licensee employing an agent, procurer, contractor, or employee for purposes of soliciting new patients shall file, in a format approved by the board, a registration form an a copy of the procurers' driver's license or state issued ID before the procurer acts on the chiropractor's behalf. Each procurer registration shall terminate on December 31. The chiropractic physician shall register each procurer annually. The chiropractor is required to provide the board with updated procurer registration information, should any of it change during the year. All registered phone numbers and pictures of the procurers will be made public on the board's website to be as transparent as possible. The chiropractic physician is responsible to the Board for the content of the contact, including prohibited statements made or required statements not made, as well as for any action that is foreseeable in a telephone or in-person encounter.
(1) Telephone solicitation/marketing of victims of accidental injury and which are conducted on behalf of chiropractic physicians shall be made in substantial conformance to a written script which is considered by the Board to have been specifically approved by the chiropractic physician. The chiropractic physician shall be required to maintain such scripts for a period of two (2) years following their utilization. Scripts are to be made available for review upon request by this Board or its designee.
(2) Agents, procurers, contractors, or employees of chiropractic physicians who solicit victims of accidental injury shall keep a log of all solicitation calls made, including at minimum the name and phone number of the person being solicited, the date and time of the phone call.
ASBCE - Continuing Education
License Information
AR Approved Continuing Education
Upcoming CE courses for 2023:
The CE rosters are in order by the sponsor name and each sponsor begins on a new page. You can scroll through the document or you can do a search/find (Ctrl + f) to look for something specific. Also provided are rosters by the course start date for easier access to what is coming up each month.
Continuing Education - Licensure Requirements
PLEASE NOTE: It is the responsibility of the chiropractor to obtain the certificate of attendance and mail it to the Board office with the license renewal during the license renewal period.
In compliance with provisions of the Arkansas Chiropractic Practices Act, each licensed Doctor of Chiropractic, practicing in this State, must annually submit evidence to the Arkansas State Board of Chiropractic Examiners of having satisfactorily completed not less than twenty-four (24) hours of continuing education, acquired during the preceding twelve (12) months, conducted by an approved chiropractic college, institution or at the approved educational course, program or seminar. A maximum of twelve (12) distance-based learning credit hours may be submitted by a licensee during each licensing period. See Rules and Regulations Section E(3).
-
Courses, programs, or seminars must be pre-approved by the Arkansas State Board of Chiropractic Examiners.
-
Speakers or lecturers must be recognized as having expertise in the field of study.
-
The course, program, or seminar must be conducted by a recognized and reputable school, university, hospital, organization, or inter-disciplinary organization.
-
Content of the course, program, or seminar must be scientific, recognized by reputable authorities as having validity, and related to the practice of chiropractic.
-
Courses, programs, or seminars taught in conjunction with, or in association with, and not sponsored and managed by an approved college or association, with their regular faculty and post-graduate instructors, will not be approved.
-
Courses, programs, or seminars conducted by colleges holding status with the Council on Chiropractic Education (CCE), or those courses, programs, or seminars sponsored by state or national associations will generally be approved provided the course content and instructional staff complies with CCE and the Board’s criteria.
-
Certification of twenty-four (24) hours of continuing education must accompany the Renewal License Application.
Sponsor Information
Application for Continuing Education Credit Approval.
The following steps must be followed when submitting a request for continuing education credit approval to the Arkansas State Board of Chiropractic Examiners:
- Continuing Education Seminar Application form
- Sponsor Contact Information (form)
- The course work must be at the physician level and the content of the program must be scientific and related to the practice of chiropractic. The AR Chiropractic scope of practice is inclusive in the Rules, Regulations, and Statutes.
- A five dollar ($5) processing fee per every credit hour of instruction for each seminar per subject material, per calendar year. The exact amount must be included; credit and refunds cannot be given.
- Applications must be submitted a minimum of 60 days before the seminar date. Any submissions after that deadline will need approval from the ASBCE office.
- Incomplete applications will be returned with an incomplete notice. If the application can be completed and mailed back to the office before the seminar date, it will be accepted and processed. If it arrives the day of, or after, the seminar date, it will not be processed and will be returned to the sponsor.
Mail the completed form, accompanying information, and processing fee(s) to:
Arkansas State Board of Chiropractic Examiners
ATTN: Continuing Education
101 East Capitol Avenue, Suite 209
Little Rock, AR 72201
ASBCE - Applicants
Chiropractic Aides | Chiropractic Student Preceptorship | Temporary License
All applications can be found and submitted here.
*DO NOT USE A Mobile devise or iPad.
ASBCE does not reciprocate with any other states.
Application for Licensure Information
NBCE Parts I, II, III, IV & the Physiological Therapeutic section are mandatory effective July 1, 2016.
The Arkansas State Board of Chiropractic Examiners offers a new doctor orientation four times a year. January, April, July, and October. The new doctor orientation is a prerequisite to licensure.
-
Must be 21 years of age.
-
Fees - made payable to the ASBCE:
- $150 application fee
- $36.25 State/FBI background check fee
- $50 orientation fee
-
Copy of Chiropractic diploma, or date you will graduate
-
Official Chiropractic transcript (Sent directly from college) to show 120 classroom hours of physiological therapeutics and not less than 4,400 fifty minute resident class hours or not less than 4 years of 9 academic months.
-
Official Undergraduate transcript(s) (Sent directly from college) Applicant must have not less than a total of sixty (60) semester hour credits, of which a minimum of thirty (30) semester hour credits must be in science subjects such as biology, zoology, chemistry, math, or other like subjects, with no less than a "C" average.
-
Official National Board Transcript (Sent directly from NBCE) to include scores for Part I, Part II, Part III, Part IV (minimum score of 375), and Physiological Therapeutic
-
Letter of recommendation (must be from a doctor of five years of experience and printed on his/her letterhead)
-
Criminal Background check
-
A completed, signed, and notarized release form and fingerprint cards (Will be mailed to the applicant upon receipt of the application)
-
Arkansas Resident Applicants:
-
Forms will be emailed upon receipt of the application
-
-
Copy of your driver’s license
-
-
Verification of licensure in good standing from all other states (s) where you are licensed or have held a license- if applicable.
-
Jurisprudence exam (Will be mailed to the applicant upon receipt of application)
-
Incomplete applications are kept on file for one year. All application fees are non-refundable.
-
All application materials including the JP exam and fingerprint packet, other than the National Board Part IV score, must be received by the Executive Director of the ASBCE with a postmark or electronic receipt of no later than 45 days prior to the orientation date. The National Board Part IV score must be in the hands of the Executive Director of the ASBCE with the postmark no later than 7 days before the orientation date.
Applicants that have NOT graduated from Chiropractic College:
-
An approved applicant will be permitted to sit for this Board's orientation provided the applicant's date of graduation from Chiropractic College precedes the date of the next regularly scheduled examination by no more than six (6) months.
-
Upon graduation, you must request that the college mail to our office an official transcript and a copy of your diploma. Your license will be held until proof of graduation is received.
If you are an Active Military member stationed in Arkansas or a Veteran or the spouse of one, please submit the Military Expedite form with your license application. You may need to upload this form to your online application.
Please complete the License Application and if remitting payment by check, mail fees to::
ASBCE
101 East Capitol Ave., Ste. 209
Little Rock, AR 72201
Chiropractic Aides
Ark. Code Ann. § 17-81-102(4) 'Chiropractic aide' means an unlicensed member of the chiropractic team who may assist a chiropractic physician in the performance of those procedures and techniques constituting the practice of chiropractic as defined in this chapter with the exception of spinal manipulation and adjustment, provided that such assistance shall be performed under the direct supervision of a licensed chiropractic physician.
Code Ark. R. 029.00.2-E(2)
- (a) The chiropractic aide may not render any diagnosis, submit treatment plans to patients, or in any other way assume responsibility for the management of patient care.
- (b) The chiropractic aide may not render any manipulative adjustment treatment or spinal mobilization.
- (c) The chiropractic aide may perform specific testing procedures and/or adjunctive therapeutics under the order, direction and responsibility of the supervising doctor.
- (d) Chiropractic aides must obtain a Radiologic Technology License through the Arkansas Department of Health to perform x-rays. The Consumer-Patient Radiation Health and Safety Act, Act 1071 of 1999, requires that individuals who use radioactive materials or medical equipment emitting or detecting ionizing radiation on human beings for diagnostic or therapeutic purposes, be licensed to do so.
- (e) In lieu of obtaining a Radiologic Technology License under subpart (d), chiropractic aides may obtain certification through the American Chiropractic Registry of Radiological Technologists (ACRRT) program upon successfully completing a course of instruction consisting of didactic classroom hours and examination. The course must be recommended by the Board. ACRRT recertification requires a minimum of 6 hours of continuing education administered by a state or national organization approved by the Board for this purpose.
ADH Radiologic Technology
ACRRT
Chiropractic Student Preceptorship Information
The Preceptorship program has been established in order to allow chiropractic students in the final clinical phase of their training to practice under the direct, on-site, supervision of a chiropractor licensed in this state.
Preceptor(supervisor) Application to be submitted to the board can be found on the application link at the top of this page.
Temporary License Information
Fees - made payable to the ASBCE:
- $30 Temporary License Application Fee
An applicant may apply for a temporary license once a COMPLETED application for licensure is on file with the Arkansas State Board of Chiropractic Examiners. Temporary license applications are voted on by the full Board.
Supervised Temporary License
After a temporary license holder has received approval from the Board, he may perform any acts or practices that a licensed Arkansas Chiropractor may do, as long as it is under the supervision of the supervising Chiropractor who must remain on the premises when these acts or practices are being performed.
Upon satisfactory evidence being submitted to the Board as to an applicant's ability and integrity and when no regular examination will be held within thirty (30) days from the date of an application for a temporary license, the board may, if approved by at least two-thirds of the membership of the Board, issue to the applicant a permit to practice until the next regular meeting of the Board.
Supervising Doctor Requirements:
- Have an active Chiropractic license of 3 years or longer.
- Not have any disciplinary action levied against him by any Board in the past 5 years.
- Have no more than two (2), temporary license holders, under his direct supervision at one time.
Unsupervised Temporary License
The Board may at its discretion, issue a temporary license to a Doctor of Chiropractic who holds a current license in another state, to practice in Arkansas until his next scheduled examination and exempt him/her from any supervisory requirement.
Please complete the Temporary License Application on the application link at the top of this page, and if paying by check, mail check to:
ASBCE
101 E. Capitol Ave., Ste. 209
Little Rock, AR 72201.
Chiropractic Externship Information
The Externship program has been established in order to allow chiropractic graduates, who are still attempting to pass their National Boards, to practice under the direct, on-site, supervision of a chiropractor licensed in this state.
Please complete the Extern and Supervisor applications on the application link at the top of this page.
ASBCE - Licensees
- ASBCE Chiropractic Guidance Update April 6, 2021
- Change of Address Form
- Reactivation of Lapsed License Application
- Reinstatement of Suspended License Request Form (Due 30 days prior to the end of suspension)
- Acupuncture
- Animal Chiropractic
ASBCE - Meeting Minutes
Board Meeting Minutes:
- January 19, 2023
- December 6, 2022 Special Meeting Teleconference
- October 20, 2022
- September 19, 2022 Special Meeting Teleconference
- July 21, 2022
- May 23, 2022 Special Meeting Teleconference
- April 14, 2022
- March 28, 2022 Special Meeting Teleconference
- February 11, 2022, Special Meeting Teleconference
- January 20, 2022 Teleconference/Video Conference
- December 9, 2021, Special Meeting Teleconference
- November 4, 2021
- September 7, 2021, Special Meeting Teleconference
- July 20, 2021 In-person/Video Conference
- June 24, 2021 Teleconference
For minutes older than 2021, contact the Board's office.
Arkansas State Board of Chiropractic Examiners
The State Board of Chiropractic Examiners was created by Act 126 of 1915, as a regulatory board to:
- Provide for the licensing and examination of chiropractors.
- Ensure that any person practicing or offering to practice chiropractic in the state is qualified and licensed.
- Safeguard the public health and welfare of the citizens of Arkansas.
The Arkansas State Board of Chiropractic Examiners provides access to resources and other information on this Web Site as a public service. Although reasonable efforts have been made to ensure that all electronic information made available is current, complete, and accurate, the ASBCE does not warrant or represent that this information is current, complete, and accurate. All information is subject to change on a regular basis, without notice. The Agency assumes no responsibility for any damages incurred as a consequence, directly or indirectly, of the use and application of any of the contents of this site.
Calendar: Below are the dates of meetings and deadlines for application materials.
| Board Meetings: To request an item be included on a board meeting agenda, please submit the request in writing to the Board office. | ||
| Date | Time | Agenda Item Request Due |
| July 16, 2024 | 9am | tbd |
| Deadlines for all application documents (including background check forms) | ||
| tbd | ||
| Staff |
| Elizabeth Jones, Director |
| Board Members |
| Note: Please call the Board office for any questions you might have. (501) 682-9015. |
File a Complaint:
The Chiropractic Board is empowered by law to enact, interpret, and apply rules and regulations governing the conduct of individuals licensed under the State of Arkansas Chiropractic Practice Act.
Any person or legal entity may file a complaint or report a violation to the board. Such complaints may be submitted in writing, along with the ASBCE Uniform Complaint Form, and should state facts, which indicate possible misconduct by the licensee. The board may act on its own initiative if evidence of misconduct comes to the attention of the board.
A complaint may also be filed through the online complaint portal HERE.
When filing a complaint give full details, which should include facts, details, and dates. Attach all billing document records, correspondence, and contracts.
If the doctor is licensed by the Arkansas State Board of Chiropractic Examiners a letter is mailed to the doctor along with a copy of the complaint requesting a response within ten days. Upon receiving a letter of response from the doctor the investigation committee determines whether further investigation is warranted or whether an informal disposition may be attempted by settlement, consent, agreement, or for lack of sufficient probable cause.
Upon completion of an investigation, the Board investigation committee determines whether a disciplinary hearing should be scheduled to resolve the issue.
The licensee has a right to a fair hearing. Procedures, which protect the licensee's rights, while allowing the board to conscientiously enforce its rules, are essential to an effective disciplinary environment.
Resources:
- Rules
- Meeting Minutes
- Freedom of Information
- Professional Associations and Organizations related to or used by Chiropractors
- Arkansas Student scholarship for Chiropractic College (Scholarships may be available for Arkansas residents to attend chiropractic college)
- State Holidays/Office Closings (Legal Holidays by Authority of Act 304 of 2001)
- Roster
- Disciplinary Actions
- Procurer Roster (actively registered for 2023)
- FAQs
- Arkansas Financial Transparency
| Office | Address | Phone |
|---|---|---|
| Arkansas State Board of Chiropractic Examiners | 101 East Capitol Avenue, Suite 209 Little Rock, AR 72201 |
(501) 682-9015 Business (501) 682-9016 Fax (currently not working) (866) 257-8277 Toll Free |
PANS/PANDAS
What is PANDAS?
According to the National Institutes of Health, PANDAS is short for Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections. A child may be diagnosed with PANDAS when:
- Obsessive-compulsive disorder (OCD), tic disorder, or both suddenly appear following a streptococcal (strep) infection, such as strep throat or scarlet fever.
- The symptoms of OCD or tic symptoms suddenly become worse following a strep infection.
The symptoms are usually dramatic, happen “overnight and out of the blue,” and can include motor or vocal tics or both and obsessions, compulsions, or both. In addition to these symptoms, children may become moody or irritable, experience anxiety attacks, or show concerns about separating from parents or loved ones.
Resources:
POLST - Resources
Questions or comments for AR POLST may be emailed to arpolst@hpcaa.org
- Click here for the current AR POLST Form download.
- Click here for a detailed video describing AR POLST
- Click here to learn about physician and nurse practitioner billing for ACP discussions
- Click here to access The Caring Conversations® Workbook for you and your family
Advance Care Planning
- UAMS Advance Directive Form: Visit Website
- The Conversation Project: Visit Website
- Prepare for Your Care: Visit Website
- Caring Info (NHPCO): Visit Website
- American Bar Association Advance Care Planning Toolkit: Visit Website
- AARP: Visit Website
POLST - Advance Directives
There are three advance care planning documents:
Advance Directive
This is a legal document that states what kinds of treatment should be given to us when we can no longer make decisions or speak for ourselves. It only goes into effect if we are terminally ill or have lost decision-making capacity, and it is usually completed in advance of any known illness. We complete it ourselves (no one else may complete the form for you), and unless there are other known facts, it must be honored. To become valid, it must include the signatures of two witnesses. All adults should have an Advance Directive.
Durable Power of Attorney for Health Care
This legal document authorizes someone chosen by an individual (called an ‘agent’) to make decisions on their behalf if they are no longer able to speak for themselves. The agent makes decisions on behalf of the patient which is aligned with their known or stated preferences for ongoing medical care.
AR POLST does not replace the above forms but can be used to operationalize the directives of the living will.
Advance Care Planning Resource for Patient & Families
The Center for Practical Bioethics shares The Caring Conversations® Workbook to guide you, your family, and your friends through the process of advance care planning with a highly individualized focus. The Workbook includes a Durable Power of Attorney for Healthcare Decisions form as well.
Click here to access.
POLST - Forms & Directions
Providers
AR POLST form is available through the Arkansas Department of Health website. Changes to the form are made through the legislative process.
Click here to download the current AR POLST form.
AR POLST (Physician Orders for Life-Sustaining Treatment) is an easily identifiable pink document that translates a patient’s goals of care and treatment preferences for end-of-life care into a physician order that transfers across AR health care settings. The AR POLST form represents a “plan of care” for a patient with a life-limiting illness and is modeled after the Physician Order for Life-Sustaining Treatment (POLST) Paradigm which is used in many states.
The document should be completed after a thorough discussion with the patient, and if the patient is incapacitated, by his/her legal health care representative* regarding the patient’s understanding of the illness, treatment preferences, values, and goals of care. Completion of an AR POLST form encourages communication between doctors and patients, enables patients to make informed decisions, and clearly documents these decisions to other physicians and health care professionals. As a result, AR POLST can help ensure that a patient’s wishes are honored, prevent unwanted or non-beneficial treatments, and reduce patient and family stress regarding decision-making.
AR POLST does not replace an advance directive for health care but can be used to operationalize the directives of the living will.
The AR POLST form is an essential part of health information records that travels with the patient as he/she moves from one health care setting to another. A copy should always be kept in the patient’s chart or medical record and be immediately accessible by other health care providers as necessary across care settings.
An AR POLST form may be revoked/voided at any time by the patient or legal proxy.
Please click here for a detailed video describing AR POLST.
Please click here to learn about physician and nurse practitioner billing for ACP discussions.
Compliance with AR POLST
In some cases, physicians have been hesitant to follow AR POLST orders without first reassessing the person’s wishes in the current clinical situation. However, the AR POLST form states that the orders on AR POLST form should be followed until a review is completed by the accepting health care professional. The AR POLST form should be followed even if the physician who has signed the document is not on the medical staff of the facility.
Health care institutions are encouraged to develop policies and procedures for the use of AR POLST.
The Impact of AR POLST
Research has shown that documents like AR POLST are making a difference in end-of-life care. It serves as a useful tool for starting a dialogue with patients regarding their wishes about end-of-life care.
Studies in states that have POLST available have revealed that among patients with completed POLST documents, treatment preferences were respected 98% of the time, and no one received unwanted CPR, intubation, intensive care, or feeding tubes. As a result, POLST has helped to bridge the gap between preferred treatment options and the actual treatments patients receive.
Heart Disease Maps
Arkansas Minority Health Commission

Registration Now Open: 8th Biennial Minority Health Summit To Be Hosted on Tuesday, April 23rd
Mission
To assure all minority Arkansans equitable access to preventive health care and to seek ways to promote health and prevent diseases and conditions that are prevalent among minority populations.
The Commission supports its mission through:
- Studying issues relating to the delivery of and access to health services for minorities in Arkansas;
- Identifying any gaps in the health service delivery system that mainly affect minorities;
- Making recommendations to relevant agencies and to the legislature for improving the delivery and access to health services for minorities; and
- Studying and recommending whether adequate services are available to ensure future minority health needs will be met.
Vision
The AMHC’s vision is that minority Arkansans have equal opportunity and access to health care and preventive well care.
Goals
Our goal is to be a catalyst in bridging the gap in the health status of the minority population and that of the majority population in Arkansas. To accomplish this, the commission focuses on addressing existing disparities in minority communities, educating these communities on healthier lifestyles, promoting awareness of services and accessibility within our healthcare system, and making recommendations to relevant agencies, the Governor, and the state legislature.
Forms
- Sponsorship instructions and Application (Monday, March 18, 2024 and closes Tuesday, April 30th at 4:30pm)
Initiatives
Upcoming Events
Resources
- Workforce Diversity Report: 2022
- Funding Opportunities
- Request a Speaker
- Schedule Mobile Health Unit
- Become a Volunteer
Key Partners
- AARP
- Healthy Active Arkansas
- Tobacco Settlement
- Million Hearts
- OMH
- ACHI
- AMDPA
- Arkansas Cancer Coalition
- Center for Healing Hearts and Spirits
| Office | Address | Phone | Fax |
| Arkansas Minority Health Commission | 5800 W. 10th St. Suite 805 Little Rock, AR 72204 |
501-686-2720 1-800-264-2826 |
501-686-2722 |
Connect With Us: Twitter | Facebook | YouTube | Instagram
Arkansas Maternal Mortality Review Committee
Purpose | Vision | Mission | Goals
The Arkansas Maternal Mortality Review Committee (AMMRC) began with the passage of Act 829 of 92nd General Assembly Regular Session, 2019. The AMMRC was developed with guidance from the Centers for Disease Control and Prevention (CDC) Building US Capacity to Review and Prevent Maternal Deaths and is modeled after well-established review committees in the United States.
To improve maternal health outcomes, you must begin with understanding the factors that contribute to maternal mortality. The Arkansas Department of Health (ADH) established inter-and intra-agency agreements to identify maternal deaths and to access records to facilitate committee review. Through data collection and analysis of clinical factors, preventability, and social determinants, the AMMRC seeks to identify factors that lead to poor maternal health outcomes and to make recommendations that will decrease maternal mortality and morbidity.
to facilitate committee review. Through data collection and analysis of clinical factors, preventability, and social determinants, the AMMRC seeks to identify factors that lead to poor maternal health outcomes and to make recommendations that will decrease maternal mortality and morbidity.
Membership
The AMMRC is a multidisciplinary committee whose members represent the state of Arkansas. The members consist of various specialties, facilities, and systems that interact with and impact maternal health.
The members are appointed by the Arkansas Secretary of Health and consist of specialists in obstetrics and gynecology, maternal-fetal medicine, anesthesiology, nursing, psychiatry, mental/behavior health, nurse midwifery, public health, hospital association, patient advocacy, and more. Recruitment of new AMMRC members occurs as needed.
All AMMRC members serve in a volunteer capacity and do not receive compensation for participation in the review process. ADH Family Health Branch maintains an internal workgroup to provide administrative support for the committee.
Resources
- Correct Way to Wear a Seatbelt During Pregnancy | Espanol
- National Maternal Mental Health Hotline
- Urgent Maternal Warning Signs
- HEAR HER Campaign | CDC
- Review to Action
- Suicide Prevention and Education
- Substance Misuse Education and Prevention
- Violence Prevention and Education
Resources for Committee Members
- MMRIA Login Assistance
- AMMRC Guidance Document
- Confidentiality Statement for AMMRC
- MMRIA
- MMRIA Committee Decision Form
- AMMRC Committee Decision Tool
Reports
- AMMRC Legislative Report 2023
- AMMRC 2023 Fact Sheet (2018-2020 Data)
- AMMRC Legislative Report 2022
- AMMRC Legislative Report 2021
- AMMRC Legislative Report December 2020
What is maternal mortality?
In understanding maternal mortality or deaths, several terms exist depending on what and who the term is being used for (i.e., surveillance and reporting, public education, grant applications, etc.). Below are a few common terms and definitions:
- Maternal death: The death of a woman while pregnant or within 42 days of termination of pregnancy, irrespective of the duration and the site of the pregnancy, from any cause related to or aggravated by the pregnancy or its management, but not from accidental or incidental causes.
- Maternal mortality rate: The number of maternal deaths per 100,000 live births.
- Pregnancy-related mortality ratio: The number of pregnancy-related deaths per 100,000 live births.
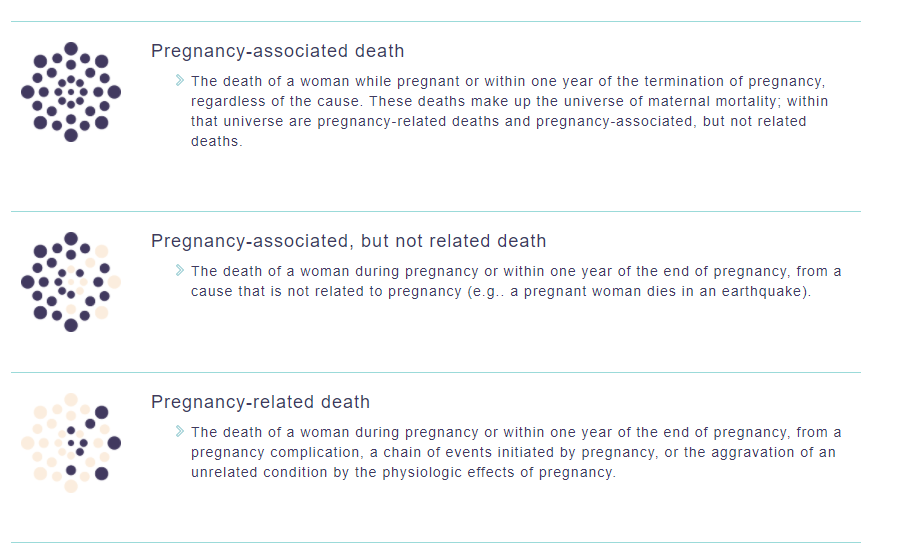
Scope
The scope of cases for Arkansas review is all pregnancy-associated deaths or any deaths of women during pregnancy or up to 365 days after pregnancy ends. In July 2020, committee members set forth exclusion criteria for abstraction (i.e., motor vehicle accidents and out-of-state residents). Deaths are identified from a review of death certificates, based on the cause of death, a pregnancy check box selection, or linkage of vital records by searching death certificates of women of reproductive age and matching them to birth or fetal death certificates in the year of or the year prior to the woman’s death.
The purpose of the AMMRC is to identify and characterize maternal deaths with the goal of identifying prevention opportunities.
To protect and improve the health and well-being of all Arkansans by eliminating preventable maternal deaths in Arkansas.
Optimize health for all Arkansans to achieve maximum personal, economic, and social impact.
The goals of the AMMRC are to:
- Perform thorough record abstraction to obtain details of events and issues leading up to a mother’s death.
- Perform a multidisciplinary review of cases to gain a holistic understanding of the issues.
- Determine the annual number of maternal deaths related to pregnancy (pregnancy-related mortality).
- Identify trends and risk factors among pregnancy-related death in Arkansas.
- Recommend improvements to care at the individual, provider, and system levels with the potential for reducing or preventing future events.
- Prioritize findings and recommendations to guide the development of effective preventive measures.
- Recommend actionable strategies for prevention and intervention.
- Disseminate the findings and recommendations to a broad array of individuals and organizations.
- Promote the translation of findings and recommendations into quality improvement actions at all levels.
Quarterly Meetings
The AMMRC meets quarterly to review select cases and make recommendations about each case based on the case narrative and abstracted data. These are closed meetings. Members of the public or press will not be allowed at AMMRC meetings. If members of the public or press show up uninvited at a meeting, they will be notified that the AMMRC meetings are not open to the public and will be asked to leave. Members of the public or press will be offered the opportunity to engage with ADH staff about the work at a separate time outside of the AMMRC meetings. The committee examines the cause of death and contributing factors. This multidisciplinary group assembled for the first official meeting in January 2020.
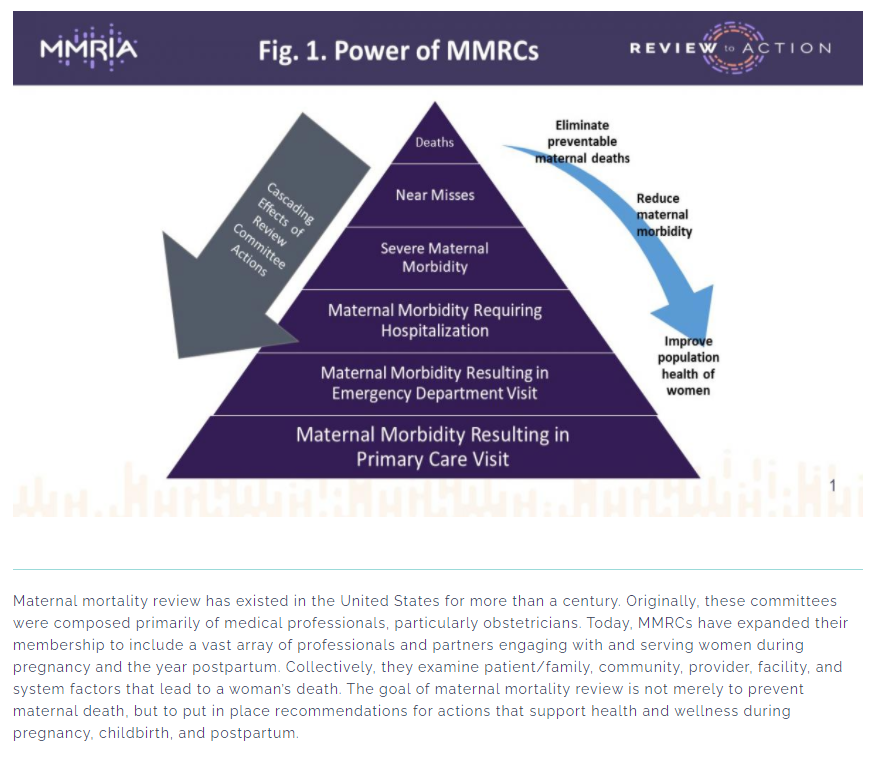
For questions, please contact: ADH-AR.Maternal.Mortality.Review@arkansas.gov
COVID-19 Vaccination Clinics & Locations
COVID-19 vaccination is recommended for everyone ages 6 months and older for the prevention of COVID-19. CDC recommends that people stay up to date with their COVID-19 vaccination.
There is no preferential recommendation for the use of anyone COVID-19 vaccine over another when more than one recommended and age-appropriate vaccine is available. COVID-19 vaccines are effective at protecting people from getting seriously ill, being hospitalized, and dying. As with other vaccine-preventable diseases, you are best protected from COVID-19 when you stay up to date with the recommended vaccinations. To get information about a vaccine provider near you see the map and table below. We recommend you contact the provider by phone ahead of time to see if the COVID-19 vaccine is available and if an appointment is needed.
You may also contact your Local Health Unit about receiving the COVID-19 vaccine.
Use the map or table below to contact your local pharmacy or provider to ask about getting the vaccine.
All Arkansas Department of Health Local Health units are COVID-19 Vaccination locations. Click here for your local health unit.
A registration code is not required by ADH.
| D.W. Reynolds Library Conference Room, 900 W Daisy L. Gaston Bates D |
COVID-19 Information On Therapeutics
Monoclonal Antibodies
Early treatment and post-exposure prophylaxis
Monoclonal antibody treatment can be used in people 12 years of age and older who weigh at least 88 pounds (40 kg) who are at high risk for severe COVID-19, including hospitalization or death for:
- Treatment of mild to moderate symptoms of COVID-19. To be eligible, patients must:
- Test positive for SARS-CoV-2.
- Be within 7 days of the start of their symptoms.
- Not be hospitalized.
- NIH statement for the treatment of COVID 19 in non-hospitalized patients.
- NIH statement to help prioritization when supplies are low.
**1/25/22, the government announced no further shipments of BAM/ETE or REGEN-COV for current and future cycles. FDA no longer recommends the use of Regeneron-COV and BAM/ETE. Please see this link for the latest update regarding therapeutics.
**4/5/2022 Sotrovimab is no longer authorized to treat COVID-19 in any U.S. region due to increases in the proportion of COVID-19 cases caused by the Omicron BA.2 sub-variant.
**NOTE**: No product returns will be accepted at this time; HHS has directed all facilities to keep their stock on hand until we receive further guidance.
Oral antivirals
Ritonavir boosted nirmatrelavir (Paxlovid) and molnupiravir (Lagevrio) were approved under EUA by the FDA for treatment of early COVID 19 infection. These treatments should be given within the first 5 days of symptom onset.
Paxlovid and molnupiravir may only be prescribed for an individual patient by physicians, advanced practice registered nurses, and physician assistants that are licensed or authorized under state law to prescribe drugs in the therapeutic class to which these drugs belong (i.e., anti-infectives).
Available COVID-19 Antivirals are covered by most insurance, typically resulting in no out-of-pocket expense.
**ORAL ANTIVIRAL TREATMENTS ARE DRIVE-THRU OR CURBSIDE ONLY! PLEASE DO NOT ENTER STORES!**
Pre Exposure Prophylaxis
A new monoclonal antibody (tixagevimab/cilgavimab) for the prevention of COVID-19 has been authorized by the FDA under EUA that may be used in immunocompromised patients and patients who have a medical contraindication to receiving a vaccine for COVID-19. Medical conditions or treatments that may result in moderate to severe immune compromise and an inadequate immune response to COVID-19 vaccination include but are not limited to:
- Active treatment for solid tumor and hematologic malignancies
- Receipt of solid-organ transplant and taking immunosuppressive therapy
- Receipt of chimeric antigen receptor (CAR)-T-cell or hematopoietic stem cell transplant (within 2 years of transplantation or taking immunosuppression therapy)
- Moderate or severe primary immunodeficiency (e.g., DiGeorge syndrome, Wiskott-Aldrich syndrome)
- Advanced or untreated HIV infection (people with HIV and CD4 cell counts
Public Health Reporting
- Click here to report COVID-19
- Communicable Disease Reporting
- Mandatory Animal Bite Reporting
- Report a Food Establishment Complaint and Feedback
CDC COVID-19 Vaccination Program Provider Agreement
Please click here or use the link below to submit a COVID-19 provider agreement form to the Arkansas Department of Health. You can also print a CDC provider agreement form here to help you gather the necessary information, but you must enter the data into the electronic form in order to submit it to ADH.
The video below has additional information about the process.
Office of Performance Management Quality Improvement and Evaluation (OPMQIE)
Performance Management System | Quality Improvement Plan | Strategic Plan
State Health Assessment | State Health Improvement Plan | Workforce Development Plan


“By improving both performance and quality, public health systems save lives, cut costs, and get better results”
– Public Health Foundation.
| Administration | |
| Deputy Chief Medical Officer | Dr. Bala Simon |
| Director | Rupa Sharma |
| Quality Assurance Analyst | Caroline Maldonado-Castro |
| Performance Management | |
| Performance Manager | Jasmin White |
| Quality Improvement | |
| Quality Improvement Manager | Patty Hibbs |
| Evaluation | |
| Public Health Evaluator | Clara Canter |
| Public Health Evaluator | Dr. Ashamsa Aryal |
Quality Improvement
OPMQIE | Performance Management System | Strategic Plan
State Health Assessment | State Health Improvement Plan | Workforce Development Plan
"Quality Improvement focuses on the process to help bring services to the next level with the aim to improve the overall health of a community." - Public Health Foundation
We can successfully achieve the goals and objectives of the Performance Management System through the commitment of all ADH staff by continually improving our work in the agency. This is referred to as a CULTURE of Continuous Quality Improvement, which is shared across all levels of the agency including Section, Branch, Center, and the executive leadership.
The CQI promises to continuously examine processes, systems, and programs for potential improvements and to implement improvement initiatives. See below for a snapshot of ADH’s QI/CQI Action Plan.
The ADH has developed a Quality Improvement Plan to guide the QI/CQI initiatives. Please click here to access the full 2020-2023 Quality Improvement Plan.
Workforce Development
OPMQIE | Performance Management System | State Health Assessment |
State Health Improvement Plan | Strategic Plan | Quality Improvement Plan
One of the attributes that help our Agency achieve the goals of the Performance Management system and its constituent components is a trained/skilled workforce. The Arkansas Department of Health, on an ongoing basis, provides training and education to its employees to enhance their skills and knowledge.
Utilizing the results of the 2017 Association of State and Territorial Officers Public Health Workforce Interest and Needs Survey as well as a SWOT (Strengths, Weaknesses, Opportunities, and Threats) analysis by an ADH internal workforce development workgroup, a comprehensive Workforce Development Plan has been developed.
The Plan roadmaps the agencies’ goals and objectives to maintain a skilled workforce that is prepared to deliver public health essential services and respond to public health emergencies. In addition to these goals and objectives, the Plan also identifies resources, roles, and responsibilities that are required to successfully implement it. Five goals (listed below) and eleven objectives for addressing training and development needs were identified.
Please click here to access the 2020-2025 Workforce Development Plan.
State Strategic Plan
OPMQIE | Performance Management System | Quality Improvement Plan
State Health Assessment | State Health Improvement Plan | Workforce Development Plan
The Interim COVID-19 Strategic Plan, 2020-2022, reflects the public health priorities of the Arkansas Department of Health to protect Arkansans during the ongoing COVID-19 pandemic.
During 2019-2020, the Arkansas Department of Health held a five-month-long exercise, involving over 70 people from the Agency and the community partners to identify public health priorities and update the 2016-2019 Strategic Plan. All employees participated in a Planning Survey to provide input on what health outcomes and health behaviors the agency should focus on. With their feedback and by using the 2020 State Health Assessment Report, the planning teams identified eight primary focus areas for the plan.
The next step of this exercise was to identify measurable objectives and work plans to operationalize the established goals. However, due to the COVID-19 pandemic, the Agency’s immediate priorities changed. The leadership decided to develop an interim plan to provide directions to mitigate the impact of the COVID-19 pandemic. However, the goals, strategies, objectives, and measures to prevent and control the COVID-19 pandemic were incorporated within the primary focus areas identified by the planning teams. This would help the Agency to make a smooth transition into developing the standard strategic plan after the pandemic is over.
The icons below represent the primary focus areas of the 2020-2022 Strategic plan.
Please click here to access the full 2020-2023 Interim Strategic Plan.
State Health Improvement
OPMQIE | Performance Management System | State Health Assessment |
Strategic Plan | Workforce Development Plan | Quality Improvement Plan
The State Health Improvement Plan (SHIP) is a long-term and systematic plan to address public health issues identified in the State Health Assessment. The goal is for the community partners to work in collaboration with its partners and the ADH to improve the health of Arkansans.
The SHIP will help partners establish priorities for the implementation of programs and policies and identify the best use of resources in achieving goals in the eight public health areas.
The Plan has been developed in a scorecard format to improve efficiency.
Please click on the icons to access the digital scorecards.
Please click here to access the State Health Improvement Plan.
State Health Assessment
OPMQIE | Performance Management System | Quality Improvement Plan | Strategic Plan
State Health Improvement Plan | Workforce Development Plan
For the first time, the State Health Assessment report is accompanied by an electronic ‘Scorecard’ which illustrates data trends for many of the issues highlighted in the report. This scorecard provides historical data to illustrate how the health issues have evolved over time and what the current trends are. Unlike the report, the Scorecard primarily presents data in graphics and sometimes tabular formats, highlighting multiple-year trends of behaviors, diseases, and deaths.
Since the Scorecard does not provide an extensive narrative and the illustrations contain layers of information, tools have been created to assist users.
A brief PowerPoint presentation provides basic guidance on how to navigate through any Scorecard.
A more detailed video provides comprehensive guidance on how to navigate through the State Health Assessment Scorecard.
Performance Management System
OPMQIE Homepage | Quality Improvement Plan | Strategic Plan
State Health Assessment | State Health Improvement Plan | Workforce Development Plan
Performance Management is a systematic process that helps an organization achieve its mission and strategic goals by improving effectiveness, empowering employees, and streamlining decision-making - Public Health Foundation.
The Performance Management System of the Arkansas Department of Health consists of the following interrelated components as shown in the diagram.
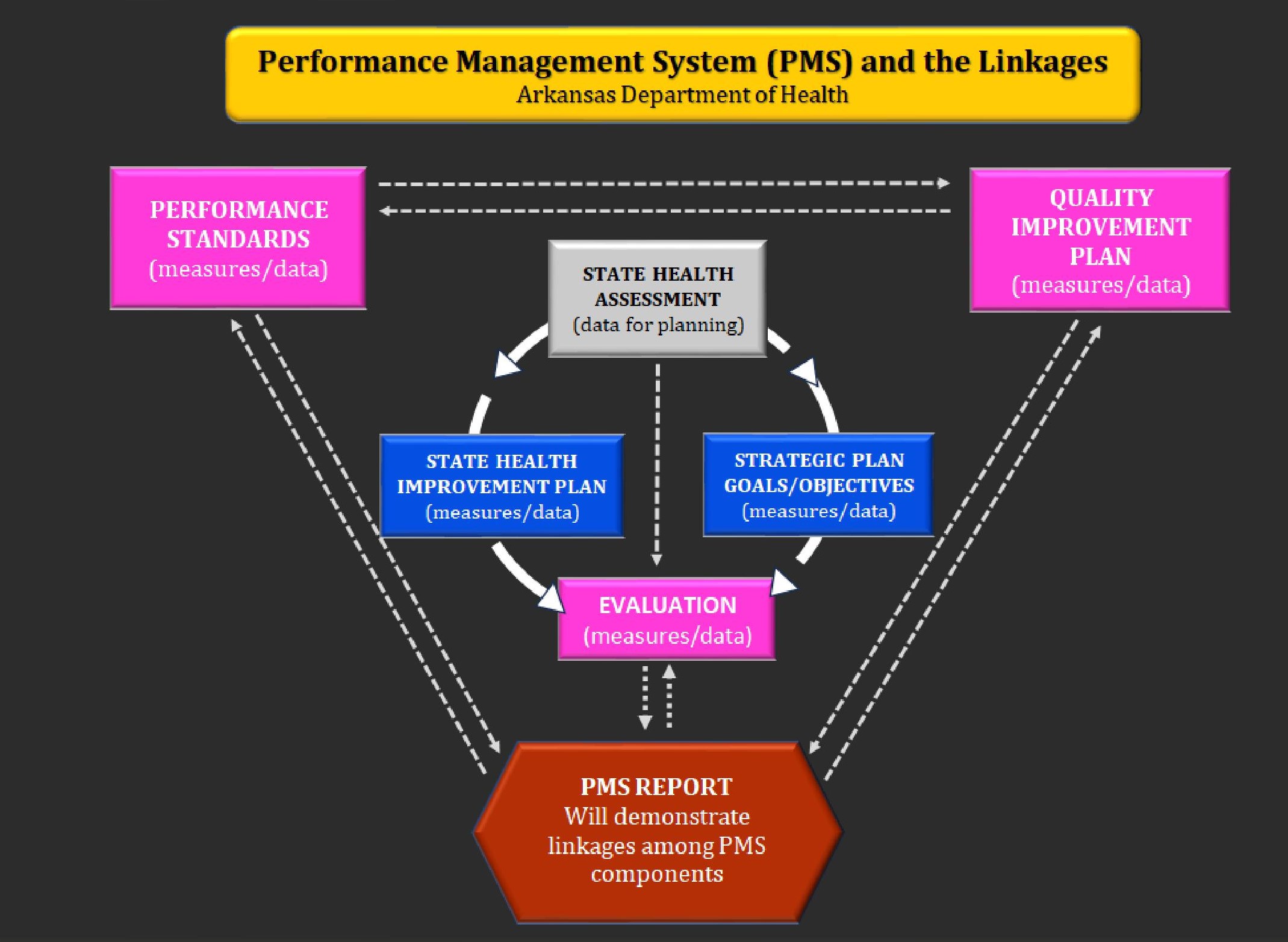
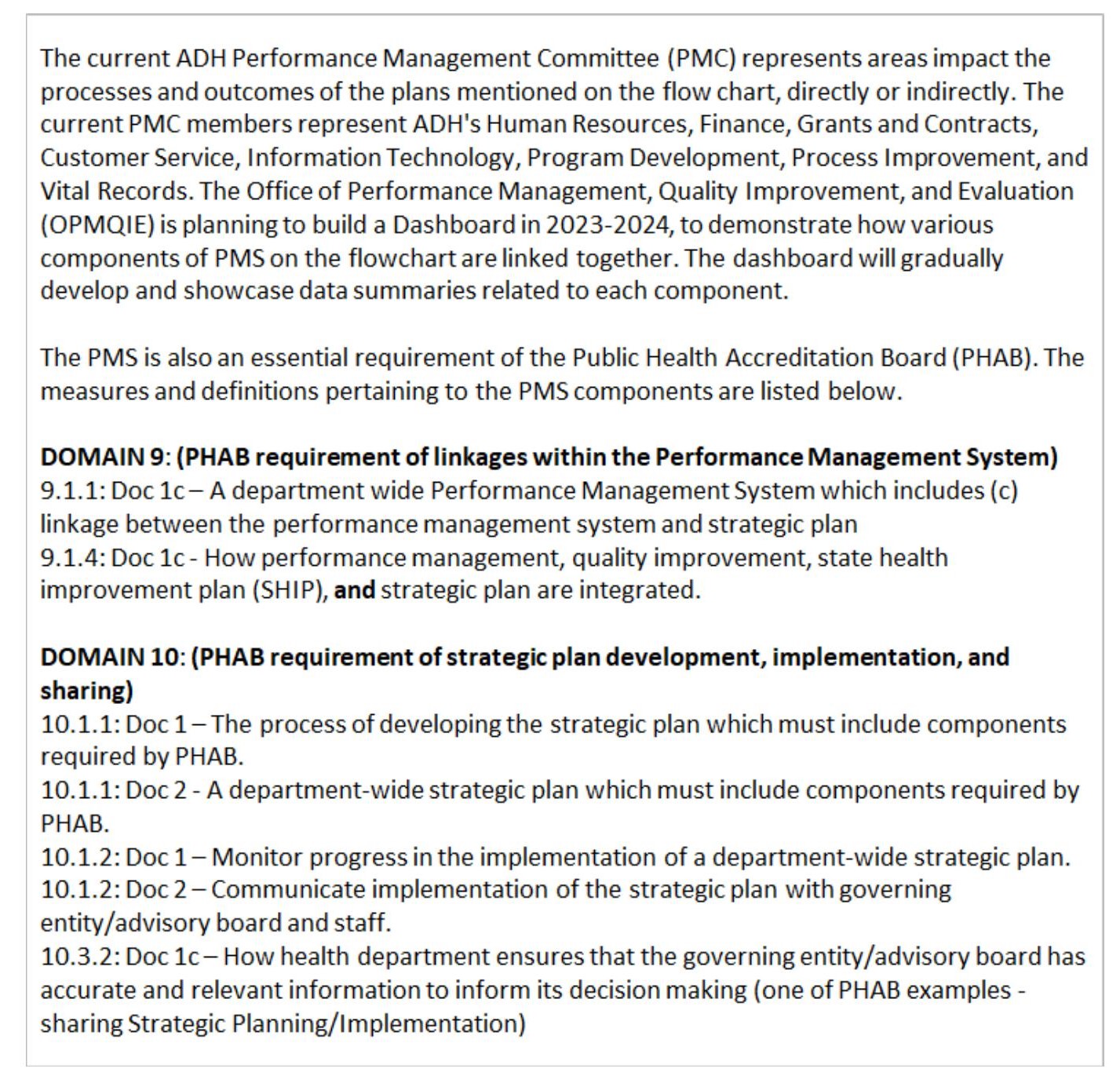
Performance Measures
Identification and use of appropriate measures are essential functions of the Performance Management System. The measures help monitor the effectiveness of programs, policies, and processes within the performance areas.
The ADH performance measures will be routinely updated by the Performance Management Committee members with new data/information, within their areas. Please refer to the performance goal areas and the active committee members listed below. The progress will be reported to the key personnel and the Public Health Accreditation Board.
Click here to find out more about the process that OPMQIE followed to select ADH’s eight performance goal areas and the measures within each area.
COVID-19 Vaccines
What do I need to know about vaccine recommendations?
- Everyone aged 5 years and older should get 1 dose of the updated Pfizer-BioNTech or Moderna COVID-19 vaccine to protect against serious illness from COVID-19.
- People who are moderately or severely immunocompromised may get additional doses of the updated COVID-19 vaccine.
- Children aged 6 months–4 years need multiple doses of COVID-19 vaccines to be up to date, including at least 1 dose of updated COVID-19 vaccine.
- COVID-19 vaccine recommendations will be updated as needed.
How can I get the vaccine?
Eligible Arkansans can make an appointment with a vaccine provider. Vaccine clinics and events may also be available in your area through local health units, pharmacies, hospitals, health care providers, or your worksite. Click here for a list of clinics.
Please see the CDC recommendations here.
- Benefits of Getting a COVID-19 Vaccine
- CDC Frequently Asked Questions about COVID-19 Vaccination
- COVID-19 Vaccine and Pregnancy | Spanish
- Pfizer-BioNTech COVID-19 Vaccine EUA Fact Sheet for Ages 6 Months to 11 Years | Spanish
- Moderna COVID-19 Vaccine EUA Fact Sheet for Ages 6 Months to 11 Years | Spanish
- Comirnaty COVID-19 Vaccine Fact Sheet
- Spikevax COVID-19 Vaccine Fact Sheet
- Vaccine Safety Monitoring
- Understanding mRNA COVID-19 Vaccines
ASBAT - Filing a Complaint
Overview of Responsibilities
The board is responsible for receiving and investigating complaints placed against athletic trainers and has authority to conduct investigations, enforce regulations and impose sanctions when a violation of law or regulation has occurred.
The Handling of Complaints
The Arkansas State Board of Athletic Training receives complaints against its licensees and will determine if the complaint falls within its jurisdiction. If the complaint is within the board’s jurisdiction, an investigation will be conducted.
The Complainant is notified of the board’s decision on each complaint. You should note, however, that the proper conduct of an investigation can be a time-consuming process and it may be several months before the investigation is completed and a decision is reached.
If a violation of the law or regulation has occurred, the board may give the licensee an opportunity to come into compliance with the law or regulation, or the board may determine that other action is necessary. If there is no violation of law or regulation, the file on the complaint is closed.
If the investigation should result in a formal hearing, the board may subpoena persons to testify at that hearing if it is believed that their testimony is essential to the case.
Filing a Complaint
Complaints should be submitted on the complaint form. It is important that you include as much fact as is available, including such things as the date(s) of the alleged action, the licensee’s full name and address, the exact nature of the complaint, the names of other individuals who might be involved and their relationship to the complaint, as well as any other information which will assist in the investigation.
The board will acknowledge receipt of your complaint, may contact you for additional information and will notify you of the board’s decision concerning the complaint.
ASBAT - Licenses and Forms
Licenses:
Forms:
- Change of Address/Change of Name
- Complaint Form
- Frequently Asked Questions
- License Verification
- NATABOC Verification
- Reinstatement Form
- Renewal Form
- Inactive Status Request
Arkansas State Board of Athletic Training
File a Complaint | Licenses & Forms
The Arkansas State Board of Athletic Training was created by Act 1124 of 2001. Prior to that time, a committee appointed by the Governor worked with the Arkansas State Board of Physical Therapy to license and regulate the activities of Athletic Trainers.
The Board is a licensing and regulatory organization created to issue licenses for athletic training and to uphold the standards prescribed by statute to protect the health of the public.
Roster:
Resources:
- Frequently Asked Questions
- Uniformed Services Member Licensure
- Renew Athletic Training License Online
Rules:
Links:
- Arkansas Athletic Trainers' Association
- The Official Website for the State of Arkansas
- National Athletic Trainers’ Association
- NATA Board of Certification
- The Southwest Athletic Trainers' Association
The Board is composed of five members appointed by the Governor for terms of three years. Four members are athletic trainers licensed to engage in athletic training in the state of Arkansas. One member is a consumer who is not actively engaged in or retired from the practice of athletic training, medicine, physical therapy, or employment by an athletic department of an accredited educational institution. This member is a full voting member.
| Board Members |
| Ronald J. Carroll, AT, Chairman |
| Terry DeWitt, AT, Secretary/Treasurer |
| Sherry Riggins, AT |
| Timothy Atkinson, AT |
| Russell Burns, Executive Director |
Upcoming meetings are posted here.
Minutes: For Board minutes earlier than 2019, contact the Athletic Training Board office:
- March 11, 2024
- October 18, 2023
- March 13, 2023
- September 12, 2022
- March 14, 2022
- November 13, 2021
- May 24, 2021
- January 29, 2021
- August 23, 2019
| Staff |
| Russell Burns, Director |
| Office | Address | Phone |
| Arkansas State Board of Athletic Training | 4815 W. Markham St., Slot 73 Little Rock, AR 72205 |
(501) 683-4076 |
ASBPT - Filing a Complaint
Overview of Responsibilities
The board is responsible for receiving and investigating complaints placed against physical therapists and physical therapist assistants and has authority to conduct investigations, enforce regulations and impose sanctions when a violation of law or regulation has occurred.
The Handling of Complaints
The Board of Physical Therapy receives complaints against its licensees and will determine if the complaint falls within its jurisdiction. If the complaint is within the board's jurisdiction, an investigation will be conducted.
The Complainant is notified of the board's decision on each complaint. You should note, however, that the proper conduct of an investigation can be a time-consuming process and it may be several months before the investigation is completed and a decision is reached.
If a violation of the law or regulation has occurred, the board may give the licensee an opportunity to come into compliance with the law or regulation, or the board may determine that other action is necessary. If there is no violation of law or regulation, the file on the complaint is closed.
If the investigation should result in a formal hearing, the board may subpoena persons to testify at that hearing if it is believed that their testimony is essential to the case.
Filing a Complaint
Complaints should be submitted on the enclosed complaint form. It is important that you include as much fact as is available, including such things as the date(s) of the alleged action, the licensee's full name and address, the exact nature of the complaint, the names of other individuals who might be involved and their relationship to the complaint, as well as any other information which will assist in the investigation.
The board will acknowledge receipt of your complaint, may contact you for additional information, and will notify you of the board's decision concerning the complaint.
Arkansas State Board of Physical Therapy
Disciplinary Action | Complaint | Continuing Education | Forms & Links |
License Verification | Online Services | Rules
Physical Therapy Licensure Compact
Information regarding the availability of compact privileges and the procedure for applying for a privilege is available online at www.ptcompact.org.
Board Information
The Board consists of five members appointed by the Governor for three-year terms. Four of those members are to be from each congressional district in order to provide a statewide representation of physical therapists. One member shall not be engaged in or retired from the practice of physical therapy and shall serve as the representative of the public interest. This is a voting member but shall not participate in examination administration.
The Arkansas State Board of Physical Therapy was created by Act 1232 of 1991. Prior to that time licensing was by the Arkansas Medical Board.
The Board is a licensing and regulatory organization created to protect the public from incompetent, unprofessional, and unlawful practice of physical therapy. Laws are established to set forth standards for the practice of physical therapy, continuing education, and testing, and to define the scope and limitations of practice. The board receives, investigates, and adjudicates complaints against licensees. The most important function of the board is consumer protection.
| Board Members |
| Joe Farrer, PT Member |
| Velvet Medlock, PT Member |
| Rob Jordan, PT Member |
| Bo Renshaw, PT Member |
| Don Pierce, Public Member |
| Michael Bynum, Attorney for the Board |
| Staff Members |
| Nancy Worthen, Executive Director |
| vacant, Administrative Specialist III |
Minutes: For board minutes earlier than 2021, contact the ASBPT office.
- January 11, 2024
- October 26, 2024
- August 24, 2023
- July 6, 2023
- June 20, 2023
- April 20, 2023
- April 14, 2023
- February 23, 2023
- February 16, 2023
- December 20, 2022
- November 3, 2022
- August 25, 2022
Board Meetings:
- tbd
- ASBPT Compact Proposed Rules
- ASBPT Rule
- Physical Therapy Telehealth Rule
- Practice Act
- Practice Act - Compact Section
Resources:
Please select the form below to view, download, or print:
- ADA Request Form
- CE Sponsor
- Continuing Education Update
- Physical Therapy Complaint Form
- Reciprocity App and Instructions
- Exam App and Instructions
- Inactive Status Request Form
- Information Change Form
- Licensure List Request Form
- Military Licensure Form
- Reinstatement Application
- Renewal Form
- Fee Waiver
Links
- CE Broker
- PT Compact Rules
- Federation of State Boards of Physical Therapy
- American Physical Therapy Association
- Arkansas State Website
- Prometric Testing Center
- Arkansas Physical Therapy Association
- Transparency.Arkansas.gov
From May 28, 2009 to Present
- Hannah Christmas
- Miranda Cohn
- Blake Pepper
- Marci Chronister
- Christian Huskins
- Hannah Harris
- Blake Pepper
- Tristan Tiarks, Revocation
- Jimmie Holland, Consent
- Megan Hausman, Consent
- Christopher Marshall, Consent
- Jeremy Bergman, Consent
- Jeremy Bergman, Revocation
- Tristan Tiarks, Suspension Order
- Tristan Tiarks, Findings
- Neely Richardson, Consent
- Jennifer Clark, PTA
- Jennifer Clark, Consent
- Charles Carter, Findings
- Benjamin Smith, Consent
- Tristan Tiarks, Order
- Wyatt Kyle, Consent
If you did not find the licensee you were looking for please check the Physical Therapist Compact Commission Verification Webpage to see if the Physical Therapist or Physical Therapist Assistant holds a compact privilege in Arkansas. For the Physical Therapist Compact Commission Verification Webpage, please click https://purchase.ptcompact.org/Verify.
| Office | Address | Phone | Fax | |
| Arkansas State Board of Physical Therapy |
Mailing: Physical: |
(501) 228-7100 | (501) 228-0294 | arptb@arkansas.gov |
ABESPA - Disciplinary Action
What is the Arkansas Board of Examiners for Speech-Language Pathology and Audiology (ABESPA)?
The Arkansas Board of Examiners for Speech-Language Pathology and Audiology (ABESPA) was created by the Arkansas Legislature to safeguard the public’s health, safety, and welfare. ABESPA is responsible for consumer protection through the regulation of speech-language pathology and audiology practice.
Individuals who seek the provisions of speech-language pathology and audiology services are entitled to receive quality care. In pursuit of that goal the Board’s mission is to assure a process is provided by which consumers may file complaints against licensees or persons practicing speech-language pathology or audiology without a license.
Who can/should file a complaint?
A complaint should be filed by anyone who believes that an individual has acted illegally, irresponsibly, or unprofessionally in providing care to a client. The most effective complaints are those that contain verifiable, firsthand information. The Board cannot act on anonymous complaints. To that end all complaints must include a signature.
What types of complaints does ABESPA handle?
- Violations of the Code of Ethics
- Violations of the Rules and Regulations
- Violation of the Practice Act
- Unlicensed Practice
What types of complaints are outside of ABESPA’s jurisdiction?
- Fee or billing disputes
- Personal conflicts
- Persons not licensed by our Board
How do I file a complaint?
A complaint must be submitted in writing and signed before it can be processed. Individuals who file complaints are notified in writing of the status of their complaint throughout the process.
When submitting a complaint, a statement should be provided in the individuals own words, which explains the nature of their complaint. Provide as much detail as possible as well as any copies of documents, such as patient records, photographs, contracts, correspondence, etc. that can be used as evidence. Original copies should not be provided. Dates, times, and the type of service received should be included when possible. It is not necessary to cite sections of the law that have allegedly been violated.
How are complaints processed?
Notification of the receipt of the complaint will be mailed to the complainant. While confidential and not public record, the board must disclose information to the licensee regarding the complaint. Licensees who are named in the complaint are given 30 days to respond to any accusations.
Complaints are reviewed by board members at the next regularly scheduled meeting. The board may dispose of the complaint informally through correspondence or conference or may forward the complaint to an investigation officer.
If forwarded to an investigator, that investigator may contact the complainant during the course of the investigation.
Are licensees required to report unprofessional conduct by colleagues?
The ABESPA Code of Ethics mandates that the licensee is responsible for reporting alleged misrepresentation or violation of the act or rules to the Board.
Resources:
ABESPA - Consumer Information
HOME | LICENSEES | APPLICANTS | CONSUMER INFORMATION | ABOUT
LICENSE SEARCH | ONLINE CE INPUT | ONLINE LICENSE RENEWAL
What is the Arkansas Board of Examiners for Speech-Language Pathology and Audiology (ABESPA)?
The Arkansas Board of Examiners for Speech-Language Pathology and Audiology (ABESPA) was created by the Arkansas Legislature to safeguard the public’s health, safety, and welfare. ABESPA is responsible for consumer protection through the regulation of speech-language pathology and audiology practice.
Individuals who seek speech-language pathology and audiology services are entitled to receive quality care. In pursuit of that goal, the Board’s mission is to assure there is a process where consumers may file complaints against licensees or persons practicing speech-language pathology or audiology without a license.
What does the Board Do?
- The Board issues license to those in compliance with the requirements in Section 2 of the rules and regulations.
- Receives and investigates complaints from the public and licensees
- Conduct settlements and disciplinary hearings of licensees
- Per federal requirements, report disciplinary action to National Data Banks
- Randomly audit licensees to ensure compliance with Section 9 of the rules and regulations and provide feedback and instruction to those audited
- Provide guidance to licensees
- Enact rules and regularly review those rules in to ensure they remain current
- Serve as a resource for licensees, applicants and the public
Audiologists
Who is an Audiologist?
Audiologists are healthcare professionals who provide patient-centered care in the prevention, identification, diagnosis, and evidence-based treatment of hearing, balance, and other auditory disorders for people of all ages. Hearing and balance disorders are complex with medical, psychological, physical, social, educational, and employment implications. Those hearing and balance disorders can be assessed, treated, and rehabilitated by an audiologist. Treatment services require audiologists to have knowledge of existing and emerging technologies, as well as interpersonal skills to counsel and guide patients and their family members through the rehabilitative process. Audiologists provide professional and personalized services to minimize the negative impact of hearing and balance disorders, leading to improved outcomes and a higher quality of life.
What does an Audiologist do?
- Evaluate and treat tinnitus, and hearing and balance disorders
- An audiologist will use a wide variety of instruments to test patients’ hearing and balance, determine the extent of hearing loss, and identify the underlying cause.
- Program and manage cochlear implant technology and other implantable devices
- Select, program, and custom-fit hearing aids and other assistive hearing technology
- Measure the effectiveness of hearing aids and other assistive technologies
- Provide counseling and education about hearing and balance disorders, hearing loss and the prevention of hearing loss
Requirements for Licensing
- Earn a doctor of audiology degree
- Obtain a passing score on the National certification exam
- Earn 10 hours of continuing education annually
Speech-Language Pathologists
Who is a Speech-Language Pathologist?
Speech-language pathologists (sometimes called speech therapists) assess, diagnose, treat, and help to prevent communication and swallowing disorders in children and adults. Speech, language, and swallowing disorders result from a variety of causes, such as a stroke, brain injury, hearing loss, developmental delay, Parkinson’s disease, a cleft palate, or autism spectrum disorders.
What do Speech-Language Pathologists do?
- Screen and assess skills in the areas of speech sound articulation, receptive and expressive language, swallowing, and hearing and recommend services if needed
- Educate clients, parents, families, and other professionals about speech, language, and swallowing
- Evaluate and assess for assistive technology when an individual does not have the ability to verbally communicate
- Work with doctors, teachers, and other professionals to help people with communication and swallowing deficits
- Research ways to improve an individual’s communication and swallowing
- Counsel patients, parents, families, and other professionals about speech, language, and swallowing
- Supervise and train students studying to become Speech-Language Pathologists
Requirements for Licensing
- A master’s or doctoral degree
- Obtain a passing score on the National certification exam
- Earn 10 hours of continuing education annually
- 400 clinical hours and 9 months of supervised experience
Speech-Language Pathology Assistants
Who is a Speech-Language Pathology Assistant?
Speech-language pathology assistants are individuals who, following academic and on-the-job training, perform tasks as prescribed, directed, and supervised by licensed speech-language pathologists.
What does Speech-Language Pathologist Assistant do?
- Conduct speech-language screenings (without interpretation) following specified screening protocols developed by the supervising speech-language pathologist
- Follow documented treatment plans or protocols developed by the supervising speech-language pathologist
- Document client progress towards goals and objectives in the established treatment plan
- Assists the Speech-language pathologist during the assessment of clients
Requirements for Licensing
- A bachelor’s degree in speech-language pathology or an associate’s degree in speech-language pathology from an institution accredited by the Arkansas Department of Higher Education
- A minimum of 100 hours of fieldwork experience
- Earn 10 hours of continuing education annually
Helpful Consumer Links
- Disciplinary Action
- American Speech-Language-Hearing Association
- American Academy of Audiology
- Arkansas Speech-Language Hearing Association
- Arkansas Academy of Audiology
ABESPA - Application Information
HOME | LICENSEES | APPLICANTS | CONSUMER INFORMATION | ABOUT
LICENSE SEARCH | ONLINE CE INPUT | ONLINE LICENSE RENEWAL
**From July 16, 2023 through July 15, 2024, the application fee is reduced from $100 to $5. This is in accordance with Act 114 of 2023. Inactive/reactivation fees are reduced from $40 to $2.
Upon initial licensure, an applicant is granted a grace period to work while his/her initial application is being processed. Upon receipt of a completed application packet, a letter will be sent to the applicant granting the grace period. Below are the licensure requirements listed by licensure type.
In order to apply for licensure, you may complete an application online then mail it in with the $100.00 application fee via check or money order, as well as the required documentation listed below. Transcripts and Verifications of Licensure must remain in a sealed envelope.
Below are the requirements listed by license type:
- Speech-Language Pathology
- Master’s degree or doctoral degree in communicative disorders
- Passing score on the PRAXIS exam or ASHA certification
- Completion of no less than 36 weeks of full-time professional experience or its part-time equivalent
- Speech-Language Pathology Provisional
- Master’s degree in communicative disorders
- Completed CFY Plan
- Appropriate clinical experience in speech-language pathology
- Speech-Language Pathology Assistant
- A Bachelor’s degree in speech-language pathology or complete a speech-language pathology assistant training program culminating in an associate degree
- At least 100 hours of fieldwork experience carrying out speech-language pathology assistant responsibilities
- Audiology
- Master’s or doctoral degree in communicative disorders
- Passing score on the PRAXIS, ASHA certification, or ABA certification
- If the applicant holds only a master’s degree, the applicant shall submit evidence of no less than 36 weeks of full-time professional experience or its part-time equivalent
Online Application Fee Payment
Important Documents:
- Graduate Student Presentation
- Clinical Fellowship Year Plan
- Application Form
- SLP Application Checklist
- Audiology Application Checklist
- SLP Provisional Checklist
ABESPA - Speech-Language Pathology Assistants
In Arkansas, a registration process is used to recognize and approve the use of Speech-Language Pathology Assistants and Aides in all settings. Individuals who wish to work as a speech-language pathology assistant or a speech-language pathology aide must register with his/her supervising speech-language pathologist with the Arkansas Board of Examiners in Speech Pathology and Audiology (ABESPA), or the Arkansas Department of Education (ADE). The setting where the Speech-Language Pathology Assistant will be used dictates which agency the individuals register with:
- Speech-Language Pathologists, Speech-Language Pathology Assistants, and Speech-Language Pathology Aides working exclusively in school districts and public early childhood programs registered with the Arkansas Department of Education.
- Speech-Language Pathologists and Speech-Language Pathology Assistants in private practice or DDS centers register with ABESPA.
- Speech-Language Pathologists and Speech-Language Pathology Assistants working in multiple sites including both private practice and school districts/early childhood programs must register with ABESPA and ADE.
Once the appropriate application is completed and approved, the names of the supervising SLP, SLP-Assistant, and SLP-Aide are placed on a registration list by each agency and all lists are submitted to ABESPA. Individuals registering with ABESPA are approved through June 30 and must re-register by July 1 each year. Individuals registering with ADE are approved through August 31 and must re-register by September 1 each year.
A registration number is assigned to the SLP-Assistant and a memo with this number is provided each time the SLP-Assistant registers or renews registration. This memo verifies that the SLP-Assistant is registered and can be submitted to Medicaid. In the future, if an individual registers with another agency, the initial registration number will be used.
Resources:
- Application Instructions to Register an SLP Assistant
- Application for Registration as a Speech-Language Pathology Assistant
- Application to Supervise SLPAs
Helpful links for SLP-A registration information:
ABESPA - Licensee Renewal Information
HOME | LICENSEES | APPLICANTS | CONSUMER INFORMATION | ABOUT
LICENSE SEARCH | ONLINE CE INPUT | ONLINE LICENSE RENEWAL
*Looking for SLPA-Related information, click here.
Renewal Information
Renewal notices are sent electronically starting in mid-April. The renewal portal opens May 1. All licenses shall be renewed annually by July 15, to avoid delinquent renewal fees. Please remember all licenses expire on June 30th of each year.
Mailed Renewals
Renewals that are mailed to the Board office will be processed as quickly as possible. Remember they must be postmarked by July 15th or be considered delinquent. If you wish to confirm that we have received your renewal documentation, we recommend sending it via certified mail.
Continuing Professional Education (CPE)
CPEs shall consist of a series of planned learning experiences beyond the educational programs that have led to the degree that qualifies one for licensure. The licensee must participate in CPE activities of at least ten (10) clock hours for each license period (July 1 through June 30 of each year). At least five (5) of these hours must be in Content Area I. Dual licensees must complete fifteen (15) clock hours with a minimum of five (5) hours in each discipline from Content Area I. Renewal of a license shall be contingent upon the licensee fulfilling the CPE requirements, submitting an annual CPE report, and maintaining evidence for possible audit.
Provisional and full licensees are not required to complete a report of continuing professional education and will not be audited during the initial licensing year, but will need to submit the report to document a minimum of ten (10) hours for the next licensing period (7/1 to 6/30). There is no exemption for the year in which the provisional license is converted to a full license unless it occurs in the initial licensing year.
Full instructions including definitions of content areas and audit information can be found in Section 9 of the rules and regulations.
Audits
Annually, the Board will select licensees for audit. Audits are randomly selected and licensees will be notified by mail. Licensees who are audited are required to submit verification of CPE’s submitted, including information regarding date, the content of course, and number of hours.
Inactive Status
Inactive status is available for licensees who wish to no longer practice in the state of Arkansas. To apply for inactive status the licensee must hold a license that is active. If inactive status is requested more than 30 days after expiration, late fees will apply. Request for inactive status must be made to the Board in writing. The individual shall not engage in the practice of speech-language pathology and/or audiology in a non-exempt facility in Arkansas while his/her/their license is inactive.
An individual wishing to regain active status shall notify the Board in writing, submit documentation of CPE’s for each year the license was inactive (maximum of 50 hours for a single license or 75 for dual licensure), and submit the $40 reactivation fee.
From July 16, 2023 through July 15, 2024, Inactive/reactivation fees are reduced from $40 to $2. This is in accordance with Act 114 of 2023.
Delinquent Renewals
Delinquent renewals will be accepted by the board through the mail. A licensee is considered delinquent if it is postmarked after July 15, and a late fee will apply. To renew after July 15, a licensee must submit the paper renewal form, the renewal fee, plus the additional late fee. The following penalties are added to the renewal fee according to the tardiness of the renewal:
- $100 between July 16-Dec. 31.
- $200 between Jan. 1-July 15
- $300 after one year of delinquency (maximum of $500.00)
Frequently Asked Questions
1. How do I make a change in my name, address, employment, or other information?
Complete the associated form, located below, and submit to abespa@arkansas.gov.
2. How do I change my SP Provisional license to a Speech Pathology license?
You may request ASHA directly send proof that you have received your Certificate of Clinical Competence, or you may forward the confirmation from ASHA to abespa@arkansas.gov. There is no additional fee or application needed for transitioning a license from provisional to Speech Pathology.
Resources:
- American Speech-Language Hearing Association
- American Academy of Audiology
- Arkansas Speech-Language Hearing Association
- Arkansas Audiology Association
Important Documents:
- CPE Self-Study Report
- Request for Address Change
- Request for Name Change
- Request for Employment Change
- Request for Status Change
Arkansas Board of Examiners in Speech-Language Pathology and Audiology
HOME | LICENSEES | APPLICANTS | ONLINE PAYMENT | CONSUMER INFO |
LICENSE SEARCH | ONLINE CE INPUT | ONLINE LICENSE RENEWAL | COMPLAINTS
Audiology & Speech-Language Pathology Interstate Compact
Information regarding the Audiology & Speech-Language Pathology Interstate compact can be found online here.
Board Information
The function of the Board is to examine the credentials and qualifications of Speech-Language Pathologists, Speech-Language Pathology Assistants, and Audiologists who wish to provide services to the people of Arkansas, and to issue licenses or registration to those individuals who meet the standards set forth in §17-100-101 et seq. The Board acts on behalf of consumers by handling complaints to ensure that quality speech-language pathology and audiology services are delivered to the public.
The Board is comprised of seven members. These include professionals in speech-language pathology and audiology (at least 2 from each field), a consumer representative, and a senior citizen representative. The members are appointed by the Governor for three-year terms and may serve two full consecutive terms. Board members may be reimbursed for expenses, but otherwise receive no compensation.
Board Members
- Colleen Sears, MS, CCC-SLP, Board Chairperson, term ends June 30, 2024
- Dr. Rebecca Rusnak, Au.D., Vice-Chairperson, term ends June 30, 2024
- Dr. Mary Chatelain, Au.D., Board Member, term ends June 30, 2025
- Aimee Cloud, MS, CCC-SLP, Board Member, term ends June 30, 2023
- Jennifer Simpson, MS, CCC-SLP, Board Member, term ends June 30, 2024
- Available Positions: Consumer Representative, Senior Representative
Staff Members
- Nathaniel Roe, Executive Director
- Bob Elder, Administrative Support Specialist
Board Meetings: All meetings are at 9am via Teams
- May 10, 2024
- July 12, 2024
- September 13, 2024
Rule
| Office | Address | Phone |
| Arkansas Board of Examiners in Speech-Language Pathology and Audiology | 4815 W. Markham, Slot 72 Little Rock, AR 72205-3867 |
501-537-9151 |
ASBN - Spouse
For purposes of Arkansas licensure, a spouse is an individual who is currently married to an active or reserve uniformed service member or uniformed service veteran.
There are several situations in which a spouse would need licensure information; each situation below identifies services available in each of these circumstances.
Situation 1:
Are you the spouse of an active or reserve uniformed service member or uniformed service veteran and do you already have a nursing license in Arkansas?
- If you answer no- advance to Situation 2 below.
- If you answered yes to both of the questions in Situation 1 and you wish to renew your active Arkansas license, click here for Renewal Instructions and note the following:
Renewal Application Information
You will submit the appropriate Renewal Licensure Application. During the process of completing your application, you will answer an eligibility question that asks “Are you or your spouse currently an active or reserve uniformed service member or uniformed service veteran?”
You will have a choice to select either “Yes, Spouse of uniformed service member” or “Yes, Spouse of uniformed service veteran”.
You will need to upload supporting documents.
Supporting documents for the spouse of a uniformed service member include one of the following:
- Copy of military dependent identification card; or
- Copy of spouses’ orders.
Supporting documents for a spouse of a uniformed veterans include one of the following:
- Copy of spouses Veterans Certificate of Release; or
- Copy of spouses’ Discharge from Active Duty form (DD214).
Support Services available in Situation 1
- Opportunity for expedited temporary permit licensure;
- Opportunity for preferential processing during issuance of license once receipt of all required documents;
- Opportunity for waiver of renewal continuing education requirements.
Situation 2:
Are you the spouse of an active or reserve uniformed service member or uniformed service veteran and do you already have a nursing license in another state?
- If you answer no- advance to Situation 3 below.
- If you answered yes to both of the questions in Situation 2:
- If your designated primary state of residence (PSOR) is a compact state and you maintain that state as your PSOR, you do not need to endorse your license to Arkansas as long as your license is an active multistate license. Advanced practice registered nurses (APRNs) are not included in the NLC. Click here for Map of Participating States.
- If your designated PSOR is a non-compact state and you maintain that state as your PSOR, you will need to endorse your license to Arkansas unless you are working in a federal facility. A nurse that works in a federal facility is exempt from licensure in Arkansas when he or she holds an active nurse license in any state.
- If you change your designated PSOR to Arkansas and you wish to apply for licensure by endorsement in Arkansas, click here for Licensure by Endorsement Instructions and note the following:
Licensure by Endorsement: Application Information
You will submit the appropriate Endorsement Application. During the process of completing your application you will answer an eligibility question that asks, “Are you or your spouse currently an active or reserve uniformed service member or uniformed service veteran?"
You will have a choice to select either “Yes, Spouse of a uniformed service member” or “Yes, Spouse of a uniformed service veteran”.
You will need to upload supporting documents.
Supporting documents for the spouse of a uniformed service member include one of the following:
- Copy of military dependent identification card; or
- Copy of spouses’ orders.
Supporting documents for a spouse of a uniformed include one of the following:
- Copy of spouses Veterans Certificate of Release; or
- Copy of spouses’ Discharge from Active Duty form (DD214).
Support Services available in Situation 2
- Opportunity for expedited temporary permit licensure, and
- Preferential processing during the issuance of a permanent license once receipt of all required documents.
Situation 3:
Are you the spouse of an active or reserve uniformed service member or uniformed service veteran? Are you a recent nursing education program graduate that has not taken the licensure examination?
- If you answered yes to both of the questions in Situation 3 and you wish to apply for initial licensure by examination in Arkansas, click here for Licensure by Examination Instructions and note the following:
Licensure by Exam: Application Information
You will submit the appropriate Initial by Exam Licensure Application. During the process of completing your application, you will answer an eligibility question that asks “Are you or your spouse currently an active or reserve uniformed service member or uniformed service veteran?”
You will have a choice to select either “Yes, Spouse of a uniformed service member” or “Yes, Spouse of a uniformed service veteran”.
You will need to upload supporting documents.
Supporting documents for the spouse of a uniformed service member include one of the following:
- Copy of military dependent identification card; or
- Copy of spouses’ orders.
Supporting documents for a spouse of uniformed include one of the following:
- Copy of spouses Veterans Certificate of Release; or
- Copy of spouses’ Discharge from Active Duty form (DD214).
Support Services available in Situation 3
- Opportunity for expedited temporary permit licensure, and
- Preferential processing during the issuance of a permanent license once receipt of all required documents.
ASBN - Uniformed Service Veteran
For purposes of Arkansas licensure, a uniformed service veteran is an individual who is a former member of the United States uniformed services discharged under conditions other than dishonorable.
There are several situations in which a uniformed service veteran would need licensure information; each situation below identifies services available in each of these circumstances.
Situation 1:
Are you a uniformed service veteran that has been discharged under conditions other than dishonorable and do you already have a nursing license in Arkansas?
- If you answer no- advance to Situation 2 below.
- If you answered yes to both of the questions in Situation 1 and you wish to renew your active Arkansas license, click here for Licensure Renewal Instructions and note the following:
Renewal Application Information
You will submit the appropriate Renewal Licensure Application. During the process of completing your application, you will answer an eligibility question that asks “Are you or your spouse currently an active or reserve uniformed service member or uniformed service veteran?”
You will select “Yes, Uniformed service veteran.”
You will need to upload supporting documents. Supporting documents include one of the following:
- Certificate of Release; or
- Discharge from Active Duty form (DD214)
Support Services available in Situation 1
- Opportunity for preferential processing during the issuance of license once receipt of all required documents; and
- Opportunity for waiver of renewal continuing education requirements.
Situation 2:
Are you a uniformed service veteran that has been honorably discharged under conditions other than dishonorable and do you already have a nursing license in another state?
- If you answer no- advance to Situation 3 below.
- If you answered yes to both of the questions in Situation 2:
- If your designated primary state of residence (PSOR) is a compact state and you maintain that state as your PSOR, you do not need to endorse your license to Arkansas as long as your license is an active multistate license. Advanced practice registered nurses (APRNs) are not included in the NLC. Map of Participating States.
- If your designated (PSOR) is a non-compact state and you maintain that state as your PSOR, you will need to endorse your license to Arkansas.
- If you change your designated (PSOR) to Arkansas and you wish to apply for licensure by endorsement in Arkansas, click here for Licensure by Endorsement Instructions and note the following:
Licensure by Endorsement: Application Information
You will submit the appropriate Endorsement Application. During the process of completing your application you will answer an eligibility question that asks “Are you or your spouse currently an active or reserve uniformed service member or uniformed service veteran?”
You will select “Yes, Uniformed service veteran.”
You will need to upload supporting documents. Supporting documents include one of the following:
Certificate of Release; or
- Discharge from Active Duty form (DD214)
Support Services available in Situation 2
- Opportunity for preferential processing during issuance of license once receipt of all required documents; and
- Opportunity for waiver of renewal continuing education requirements.
Situation 3:
Are you a recent nursing education program graduate that has not taken the licensure examination? And, are you a uniformed service veteran that has been under conditions other than dishonorable?
- If you answered yes to both of the questions in Situation 3 and you wish to apply for initial licensure by examination in Arkansas, click here for Licensure by Examination Instructions and note the following:
Licensure by Exam: Application Information
You will submit the appropriate Initial by Exam Licensure Application. During the process of completing your application you will answer an eligibility question that asks “Are you or your spouse currently an active or reserve uniformed service member or uniformed service veteran?”
You will select “Yes, uniformed service veteran."
You will need to upload supporting documents. Supporting documents include one of the following:
- Certificate of Release; or
- Discharge from Active Duty form (DD214)
Resources:
Uniformed Service Education, Training, Experience, or Service–Issued Credential
Arkansas State Board of Nursing allows an individual who has graduated from one of the listed military programs, to submit application and sit for the Licensed Practical Nurse (LPN) NCLEX®. If an individual is already licensed, refer to endorsement of a license.
- Army Practical Nurse Program
Address (and satellite tracks)
JBSA Fort Sam Houston
3490 Forage Ave, Suite 225
San Antonio, TX 78234-7585
Contact: Wilfredo E. Rivera, MAJ, AN, Deputy Director
silva.w.rivera.mil@mail.mil
Phone: 210.221.5848
Fax: 210.295.5147
School Phone: 210.295.4395
www.cs.amedd.army.mil
- Air Force Nursing Services Practical Nursing Program
Scott Air Force Base
901 South Dr
Scott AFB, IL 62225
Phone: (618) 256-4241
Support Services available in Situation 3
- Opportunity for expedited temporary permit licensure, and
- Preferential processing during issuance of a permanent license once receipt of all required documents.
ASBN - Uniformed Service Member
For purposes of Arkansas licensure, a uniformed service member is an individual who is currently serving in an active or reserve status in the United States Air Force, United States Army, United States Coast Guard, United States Marine Corps, United States Navy, United States Space Force, or National Guard; an active member of the National Oceanic and Atmospheric Administration Commissioned Officer Corps; or an active or reserve member of the United States Commissioned Corps of the Public Health Service.
There are several situations in which a uniformed service member would need licensure information; each situation below identifies services available in each of these circumstances.
Situation 1:
Are you a uniformed service member member and do you already have a nursing license in Arkansas?
- If you answer no- advance to Situation 2 below.
- If you answered yes to both of the questions in Situation 1 and you wish to renew your active Arkansas license, click here for Licensure Renewal Instructions and note the following:
Renewal Application Information
You will submit the appropriate Renewal Licensure Application. During the process of completing your application, you will answer an eligibility question that asks “Are you or your spouse currently an active or reserve uniformed service member or uniformed service veteran?”
You will have a choice to select either “Yes, Uniformed service member – I elect to Waive the Application fee” or “Yes, Uniformed service member – I elect to PAY the Application fee.”
You will need to upload supporting documents. Supporting documents include one of the following:
- Copy of military Identification card; or
- Copy of orders.
Support Services available in Situation 1:
- Option for license renewal fee waiver;
- Opportunity for preferential processing during issuance of license once receipt of all required documents;
- Opportunity for license expiration date extension for uniformed service member that is deployed outside of the state; and (Note: An Arkansas uniformed service member nurse that is being deployed outside the state can upload to nurse portal account, a request for review of expiration date extension and waiver of continuing education requirements. Also upload deployment orders.)
- Opportunity for waiver of renewal continuing education requirements.
Situation 2:
Are you a uniformed service member and do you already have a nursing license in another state?
- If you answer no- advance to Situation 3 below.
- If you answered yes to both of the questions in Situation 2:
- If your designated primary state of residence (PSOR) is a compact state and you maintain that state as your PSOR, you do not need to endorse your license to Arkansas as long as your license is an active multistate license. Advanced practice registered nurses (APRNs) are not included in the NLC. Click here for Map of Participating States.
- If your designated (PSOR) is a non-compact state and you maintain that state as your PSOR, you will need to endorse your license to Arkansas unless you are working in a federal facility. A nurse that works in a federal facility is exempt from licensure in Arkansas when he or she holds an active nurse license in any state.
- If you change your designated (PSOR) to Arkansas and you wish to apply for licensure by endorsement in Arkansas, click here for Licensure by Endorsement Instructions and note the following:
Licensure by Endorsement: Application Information
You will submit the appropriate Endorsement Application. During the process of completing your application, you will answer an eligibility question that asks “Are you or your spouse currently an active or reserve uniformed service member or uniformed service veteran?”
Please select “Yes, Uniformed service member."
You will need to upload supporting documents. Supporting documents include one of the following:
- Copy of military Identification card; or
- Copy of orders.
Support Services available in Situation 2
- Opportunity for expedited temporary permit licensure, and
- Preferential processing during issuance of a permanent license once receipt of all required documents.
Situation 3:
Are you a recent nursing education program graduate that has not taken the licensure examination? And, are you a uniformed service member?
- If you answered yes to both of the questions in Situation 3 and you wish to apply for initial licensure by examination in Arkansas, click here for Licensure by Examination Instructions and note the following:
Licensure by Exam: Application Information
You will submit the appropriate Initial by Exam Licensure Application. During the process of completing your application you will answer an eligibility question that asks “Are you or your spouse currently an active or reserve uniformed service member or uniformed service veteran?”
Please select “Yes, Uniformed service member.”
You will need to upload supporting documents. Supporting documents include one of the following:
- Copy of military Identification card; or
- Copy of orders.
Uniformed Service Education, Training, Experience, or Service–Issued Credential
Arkansas State Board of Nursing allows an individual who has graduated from one of the listed military programs, to submit application and sit for the Licensed Practical Nurse (LPN) NCLEX®. If an individual is already licensed, refer to the endorsement of a license.
- Army Practical Nurse Program
-
Address (and satellite tracks):
-
JBSA Fort Sam Houston
3490 Forage Ave, Suite 225
San Antonio, TX 78234-7585
Contact: Wilfredo E. Rivera, MAJ, AN, Deputy Director
silva.w.rivera.mil@mail.mil
Phone: 210.221.5848
Fax: 210.295.5147
School Phone: 210.295.4395
www.cs.amedd.army.mil -
Air Force Nursing Services Practical Nursing Program
Scott Air Force Base
901 South Dr
Scott AFB, IL 62225
Phone: (618) 256-4241
-
-
Support Services available in Situation 3
- Opportunity for expedited temporary permit licensure, and
- Preferential processing during issuance of a permanent license once receipt of all required documents.
ASBN - Military
Introduction
The Arkansas State Board of Nursing (ASBN) is committed to honoring the service of active and reserve uniformed service members, uniformed service veterans and their families. ASBN recognizes that these individuals experience unique challenges during the nursing licensure process. We strive to enhance the licensure process by streamlining certain services to qualified individuals. Several licensure support services are offered once all required documents are received; services include but are not limited to expedited temporary permit licensure and preferential processing during the issuance of a permanent license.
The information listed on this site identifies additional support services to assist in understanding the laws associated with respective military designation, streamlining licensure process, obtaining Arkansas licensure, and availability of other important resources.
Military Designation
In addition to the information listed on this page, click the respective link below for information related to an individual’s specific military designation status.
Associated Arkansas Nurse Practice Act (NPA) & Rules
As mandated by Act 135 of 2021, Act 820 of the 2019, and Act 248 of the 2017 Arkansas legislative sessions, the ASBN has adopted rules to assist active and reserve uniformed service members, uniformed service veterans, and their spouses in obtaining nursing licensure.
- Renewals: Option for active-duty military to have the license renewal fee waived.
- Expedited Licensure: Opportunity for expedited temporary permit licensure and preferential processing during the issuance of a permanent license once receipt of all required documents.
- Extension of Licensure Expiration Date: Opportunity for license expiration date extension for uniformed service member that is deployed outside of the state.
Note: An Arkansas uniformed service member nurse that is being deployed outside the state can upload to nurse portal account, a request for review of expiration date extension and waiver of continuing education requirements. Also upload deployment orders.
- Consideration of Military Training and Experience
- Waiver of Continuing Education: Opportunity for waiver of renewal continuing education requirements.
Click here to access Arkansas NPA & Rules in their entirety.
Licensure Portability Between States
In order to practice nursing in Arkansas, an individual is required to be licensed. Arkansas is a member of the Nurse Licensure Compact (NLC). The NLC allows for registered nurses (RNs) and licensed practical/vocational nurses (LPN/VNs) to have one multistate license, with the privilege to practice physically, electronically, or telephonically in both their home state (Primary State of Residence or PSOR) and other states that have joined the NLC. APRNs are not issued a license under the NLC.
There is specific licensure information that should be reviewed:
- What You Need to Know: Federal /Military Nurses and Spouses flyer
- ASBN NLC Information
- National Council State Boards of Nursing (NCSBN) NLC Information
Educational Opportunities
- Arkansas Nursing Education Programs
- Click here for Arkansas nursing education programs or parent institutions that offer identified services for uniformed service personnel and or veterans.
- Continuing Education Opportunities
Resources
Active and reserve uniformed service members, uniformed service veterans and their spouse can find helpful information using the links below.
- Veterans Crisis Line: Veterans Crisis Line is a free, 24/7 and confidential telephone support to Service members, National guard/Reserve, Veterans, family and friends. Veterans Crisis Line: Dial 1-800-273-8255 and press 1. Text 838255 to reach a professional responder now.
- American Council on Education (ACE) Military Guide
- Arkansas Department of Veterans Affairs
- Arkansas Division of Workforce Services
- Arkansas Military Families & Veterans Resources for Families Division of Elementary & Secondary Education
- Arkansas: My Air Force Benefits
- Arkansas: My Army Benefits
- Career One Stop
- National Archives Veterans; Service Records
- National Conference of State Legislatures (NCSL)
- NCLEX Qualified Veterans Reimbursement Program
- U.S. Department of Health and Human Services
- U.S. Department of Veterans Affairs
- U.S. Department of Veterans Affairs – Education & Training
- White House Telehealth Initiatives to Provide Greater Access to Veterans
ASBN - APRN Renewal Post Expiration (Late)
This application is for nurses who wish to renew an existing inactive/expired Arkansas APRN license. It is NOT an application for on-time renewal of new graduate initial licensure or endorsement of a license from another state. The information contained herein is designed to assist you with the information you need to begin the renewal application process.
Read the instructions and provide all required documentation. Additional instructions are located within the online renewal system as you progress through the renewal process.
General Instructions
- APRN Late Renewal Application
- No application is complete until all required documentation (if applicable) and fees are received.
- Fee Information
- Click here for current fees.
- The accepted method of payment is with credit card (Visa, Mastercard, or Discover).
- Verify that the charges are correct before submitting application.
- ALL FEES ARE NONREFUNDABLE.
- Late renewal fees are added to regular renewal fee.
- You will receive a payment receipt by email which should be printed as proof of payment.
- APRN licensure requirements include:
- Current National Certification related to you area of certification, and
- Active unencumbered Arkansas RN license OR active unencumbered multistate compact RN license.
- If out of practice greater than two (2) years a refresher course or extensive orientation is required.
- Apply or reinstate prescriptive authority:
- If you have never held prescriptive authority in Arkansas, refer to the instructions for initial prescriptive authority applicants here.
- To reinstate prescriptive authority, submit an application through your Nurse Portal account. There is no fee for this application.
- Submit the required documents:
- Collaborative practice agreement
- Quality assurance plan
- If prescriptive authority has been inactive for more than 12 months, you will need five (5) hours of pharmacology continuing education plus an additional five (5) hours for renewal including the two (2) ASBN mandatory courses. Contact the APRN Department through the Nurse Portal Message Center for more information.
- If you have been prescribing in another state within the last year, you may submit the Verification of Prescribing Hours form in lieu of the continuing education requirements.
ASBN - RN Approved Baccalaureate Degree Programs
ARKANSAS STATE UNIVERSITY
School of Nursing
P. O. Box 910
State University, AR 72467
Phone: (870) 680-8200; Fax: (870) 972-2954
www.astate.edu
Kathryn Flannigan, Chair
Phone: (870) 680-4157
Email: kflannigan@astate.edu
ARKANSAS TECH UNIVERSITY
Department of Nursing
402 W. O Street
126 Dean Hall
Russellville, AR 72801-2222
Phone: (479) 968-0383; Fax: (479) 968-0219
www.atu.edu
Dr. Shelly Daily, Chair
Email:sdaily@atu.edu
HARDING UNIVERSITY
College of Nursing
Box 12265
Searcy, AR 72149-0001
Phone: (501) 279-4476; Fax: (501) 279-4669
www.harding.edu
Dr. Susan Kehl, Dean
Email: skehl@harding.edu
HENDERSON STATE UNIVERSITY
Department of Nursing
1100 Henderson Street
Arkadelphia, AR 71999-0001
Phone: (870) 230-5379; Fax: (870) 230-5390
www.hsu.edu
Dr. Kristina Shelton, Chief Academic Nursing Officer
Email: sheltok@hsu.edu
JOHN BROWN UNIVERSITY
2000 West University Street
Siloam Springs, AR 72761
Phone: (479) 524-1662; Fax: (479) 524-7441
www.jbu.edu
Natasha Trotter, Chair
Email: NTrotter@jbu.edu
OUACHITA BAPTIST UNIVERSITY
Department of Nursing
WNEC 101
Arkadelphia, AR 71998
Phone: (870) 245-5384
Fax: (870) 245-5241
www.obu.edu
Amy Morris, DNP, RN, APRN, CPNP, CNE, Director
Email: morrisa@obu.edu
SOUTHERN ARKANSAS UNIVERSITY
Department of Nursing
100 E.University MSC9402
Magnolia, AR 71753
Phone: (870) 235-4331; Fax: (870) 235-5058
www.saumag.edu
Dr. Karen Landry. Chair
Email: KarenLandry@saumag.edu
UNIVERSITY OF ARKANSAS
Eleanor Mann School of Nursing
606 N. Razorback Road
Fayetteville, AR 72701
Phone: (479) 575-4280; Fax: (479) 575-3218
www.uark.edu
Dr. Jessie Casida, Director
Email: jcasida@uark.edu
UNIVERSITY OF ARKANSAS AT FORT SMITH
Carolyn McKelvey Moore School of Nursing
5210 Grand Avenue, College of Health Sciences Office 207
Fort Smith, AR 72913
Phone: (479) 788-7844; Fax: (479) 424-6831
www.uafs.edu
Dr. Paula D. Julian, Executive Director of Nursing
Email: Paula.Julian@uafs.edu
UNIVERSITY OF ARKANSAS AT MONTICELLO
School of Nursing
P. O. Box 3606
Monticello, AR 71656-3606
Phone: (870) 460-1069; Fax: (870) 460-1969
www.uamont.edu
Dr. Brandy Haley, Dean
Email: haley@uamont.edu
Sharon Walters, BSN Program Director
Walters@uamont.edu
Phone: (870)460-1368
UNIVERSITY OF ARKANSAS AT PINE BLUFF
1200 University Drive #4973
Pine Bluff, AR 71601
Phone: (870) 575-8217
www.uapb.edu
Dr. Brenda Jacobs, Interim Chair
Email: JacobsB@uapb.edu
UNIVERSITY OF ARKANSAS FOR MEDICAL SCIENCES
College of Nursing
4301 W. Markham, Slot 529
Little Rock, AR 72205
Phone: (501) 686-8493; Fax: (501) 686-7998
www.nursing.uams.edu
Dr. Patricia Cowan, Dean
Email: pacowan@uams.edu
Dr. Fermin Renteria
Email: RenteriaFermin@uams.edu
UNIVERSITY OF ARKANSAS FOR MEDICAL SCIENCES (NWA satellite contact)
Lauren Haggard-Duff - Dir. Accelerated BSN
Phone: (479) 713-8510
Email: lkhaggardduff@uams.edu
UNIVERSITY OF CENTRAL ARKANSAS
School of Nursing
201 Donaghey Avenue
Conway, AR 72035
Phone: (501) 450-5531; Fax: (501) 450-5560
www.uca.edu/nursing
Dr. Susan Gatto, Director
Email: Susang@uca.edu
ASBN - Post-Baccalaureate Programs
|
Contact Info |
Degrees offered |
|
Arkansas State University |
MSN |
|
Arkansas Tech University |
MSN |
|
Harding University |
MSN |
|
Henderson State University |
MSN |
|
University of Arkansas |
MSN |
|
University of Arkansas at Monticello |
MSN |
|
University of Arkansas for Medical Sciences |
MNSc |
|
University of Central Arkansas |
MSN |
ASBN - CE Broker
CE Broker is the official CE tracking system of the Arkansas State Board of Nursing. The Board has provided you with a free Basic Account, so you have their helpful education tracking tools right at your fingertips. The license renewal process and CE requirements have not changed and at this time the use of CE Broker is not mandatory.
CE Broker helps nursing professionals in Arkansas track your CE progress. This easy electronic workflow streamlines the ability for you to track your CE history and maintain your documentation or professional portfolio for personal, academic, employment, or license renewal purposes.
With CE Broker, licensees and some education providers will be able to electronically enter CE course completion certificates through the tracking system. The information is maintained within CE Broker's centralized database. This simplifies the process of tracking your continuing education with easy reporting, digital storage for your certificates, and a credit counting CE compliance transcript all in one online portal.
FREE ACCESS
CE BROKER IS 100% FREE TO USE. You will never have to pay for a CE Broker Basic Account. Licensees do have the option to subscribe to an upgraded account, which offers additional CE tracking tools outlined below.
ACCOUNT OPTIONS
ACTIVATE YOUR FREE CE BROKER ACCOUNT
- Visit www.cebroker.com/ar/plans (Works best in Chrome)
- Select the Basic Account option
- Enter your license number and get started tracking your continuing education!
If you already have a CE Broker account, follow these simple steps to add your Arkansas license.
Once you have created your account and logged into the system, click “Report CE” and find the option that best applies. You will need to answer a few questions and then attach the documentation that the Board requires. If you are using the CE Broker mobile app, you can take a picture of your documentation and upload it via your phone or tablet.
HOW TO LOCATE ADVANCED PRACTICE MANDATORY COURSES
Advanced Practice Registered Nurse (APRN) courses are located on the CE Broker platform.
Access the courses through your CE Broker account and follow these steps:
- Click on Find CE/CME
- Select filters
- In the search box, search for "Advanced practice nursing in Arkansas"
- Look for the free course with the Arkansas State Board of Nursing listed as the author
- Complete the course by clicking on the "Take It Here" button beside the course
- To find the second course, follow the steps above searching for "prescription drug abuse and misuse"
KEY FEATURES OF THE CE BROKER FREE BASIC ACCOUNT INCLUDE
Comprehensive Course Search
The CE Broker Course Search helps you find every course you need to complete your license renewal. Any Courses with the “Take it Here” tag will be instantly reported to your account and appear in your Course History. To find courses, visit courses.cebroker.com/search/ar and select your profession.
When you find CE in the Course Search, it may satisfy the requirements set by the Arkansas Board of Nursing (ASBN). The course search is not intended to be an all-inclusive list, nor are any of the vendors listed endorsed or necessarily approved by the ASBN. Depending upon the organization that approved the courses, the ASBN may or may not accept such courses for continuing education credit for the purpose of licensure renewal. Refer to the list of Accrediting Organizations/Approved Approval Bodies to determine if the provider's educational activity is accepted by ASBN. Note: You will need to be sure that the CE contact hours are practice focused relevant to your specific area of practice.
Helpful Support Center
CE Broker provides dedicated support 8AM - 8PM ET Monday - Friday with a team of experts trained on the rules and regulations of the Arkansas State Board of Nursing. You can reach them by phone at 877-434-6323 or via email and live chat.
FAQs
Visit the CE Broker FAQ directly for detailed information on CE Broker and how to use your free account.
ASBN - Medication Assistant (Certified)
Act 1423 of the 2005 Arkansas legislative session authorized the use of trained and certified medication assistants in nursing homes in Arkansas. In June 2021, the Rules were updated to authorize the use of Medication Assistants-Certified in designated facilities in Arkansas. A designated facility is defined as any Board approved facility to include a nursing home or a facility operated as a local correctional facility as defined by ACA §12-41-107. Medication Assistants are required to successfully complete an education program and pass a certification exam; or complete a portion of a nursing education program equivalent to the MA-C education program and pass a certification exam, before being allowed to function in the role of Medication Assistant-Certified (MA-C) in a designated facility.
Approved MA-C Programs
Arkansas Health Care Association
1401 W. Capitol Ave., Suite 180
Little Rock, AR 72201
Cat Hamilton
chamilton@arhealthcare.com
(501) 374-4422
Sarah Ware, Education Coordinator
Email: MACparogram@arhealth.com
Arkansas Northeastern College – Blytheville
School Code - 0011
2501 S. Division St.
PO Box 1109
Blytheville, AR 72316
Brenda Holifield, Dean
bholifield@smail.anc.edu
870-740-2908
- Kimberly Baxter, Instructor
870-740-2908
kbaxter@smail.anc.edu
Program Approved – January 2020
Next Survey Approval – January 2025
Arkansas State University - Beebe
School Code - 0013
1800 East Moore Ave
Searcy, AR 72145
Cindy Smith – Director
casmith@asub.edu
(501) 207-6255
- Program Approved – February 9, 2022
Next Survey Approval – February 2027
Arkansas State University – Mountain Home
School Code - 0006
1600 So. College
Mtn. Home, AR 72653
Amy Clark, Director
aclark@asumh.edu
870-508-6176
- Lucy Haun, Instructor
lhaun@asumh.edu
Initial Approval – May 3, 2007/
Last Survey Approval – September 14, 2017
Next Survey Approval – September 2022
Arkansas State University – Newport
School Code - 0007
7648 Victory Blvd.
Newport, AR 72112
- Dr. Stacie Hay, Dean
Stacie_hay@asun.edu
870-512-7869 - Judith Coggin, Instructor
Judith_coggin@asun.edu
870-512-7729 - Haley Thomas, instructor
Haley_thomas@asun.edu
870-512-7826
Program Approved – July 14, 2021
Next Survey Approval – July 2026
Arkansas Tech University - Ozark
1700 Helberg Lane
Ozark, AR 72949
Lisa Pittman – Head Instructor
Lpittman1@atu.edu
479-508-8500, ext 6352
- Satellite Campuses:
- ATU Career Center
- Fort Smith Adult Education Center
- Methodist Village Senior Living Center
Program Approved – February 2022
Next Survey Approval – February 2027
East Arkansas Community College – Forrest City
School Code - 0002
1700 New Castle Road
Forrest City, AR 72335
Mallory Adams, Dean of Allied Health
Email: madams@eacc.edu
Telephone:870-633-4480, ext. 452, Fax: (870) 633-7222
- Terry Davis, Director
tdavis@eacc.edu
870-633-4480 ext 470
Program Approved – September 9, 2021
Next Survey Approval – September 2026
National Park College
School Code – 0001
101 College Drive
Hot Springs, AR 71914
Janice Ivers, Dean
jivers@np.edu
501-760-4183
- Jessyca Maddox, Instructor
Jessyca.Maddox@np.edu
501-760-4340
Program Approved – January 2023
Next Survey Approval – January 2028
Northwest Technical Institute
School Code - 0010
709 South Old Missouri Road
Springdale, AR 72764
Dr. Debra Walker – Director
dwalker@nwti.edu
(479) 751-8824 ext 123
- Lisa Stockdale, Instructor
lstockdale@nwti.edu
Program Approved – November 17, 2021
Next Survey Approval – November 2026
University of Arkansas Community College – Morrilton
School Code – 0015
1537 University Blvd
Morrilton, AR 72110
Angie Moore, Chair
mooreangie@uaccm.edu
501-977-2063
Program Approved – September 8, 2022
Next Survey Approval – September 2027
University of Arkansas Pulaski Technical College – North Little Rock
School Code - 0012
3000 West Scenic Drive
North Little Rock, AR 72118
Talayia Johnson, Dean
tajohnson@uaptc.edu
501-812-2286
- Christa Jones, Director
cjones@uaptc.edu
501-812-2294
Program Approved – October 13, 2021
Next Survey Approval – October 2026
Certification
Welcome to Arkansas State Board of Nursing (ASBN). The information contained herein is designed to assist you with the information you need to begin the application process. Read the instructions and provide all required documentation. ALL FEES ARE NON-REFUNDABLE.
THE FIRST STEP in application submission is to create an account in the Arkansas Nurse Portal.
Click HERE to view the video for instructions on the CREATION OF AN ARKANSAS NURSE PORTAL ACCOUNT.
Once you have created your Nurse Portal account, sign in to your Nurse Portal account to submit an application.
Once you have submitted an application, you may monitor the status of your application by accessing your Nurse Portal account and clicking on view status.
Click HERE to view the video for instructions on How to Check Application Status.
CERTIFICATION APPLICATION INFORMATION
For more information about a specific application, click on the respective application name listed below.
Medication Assistant-Certified (MA-C) Initial Application (required for individuals that have completed Medication Assistant Certified training from an Arkansas MA-C program):
-
This Application is for an individual that has completed Medication Assistant-Certified (MA-C) training from an Arkansas MA-C program.
It is NOT an application for an Arkansas MA-C endorsement, MA-C renewal, or any type of nursing application.
The information contained herein is designed to assist you with what is needed to begin the application process. Read the instructions and provide all required documents. Additional instructions are located within the online system as you progress through the application process.
Click here for additional information located on our website at www.arsbn.org. -
General Instructions
The following 1- 4 is required:-
Application Information
-
ASBN Application
- After completing all fields on a page click the “Save and Continue” button to proceed to the next page or click the “Save and Return to Home” button if you need to exit the application and return at a later time.
- If you need to change information entered in an area, you are provided an opportunity to edit information prior to submitting the application.
- No application is complete until submitted and all required documentation and fees are received. Completed applications are valid for one year from the date of submission.
- If you are required to submit any documents, upload them through your Arkansas Nurse Portal.
-
-
Fee Information
- For current fees go to https://www.healthy.arkansas.gov/programs-services/topics/arsbn-fees
- The accepted method of payment is with a credit card (Visa, MasterCard, or Discover).
- Verify that the charges are correct before submitting an application.
- ALL FEES ARE NON-REFUNDABLE.
- You will receive a payment receipt by email which should be printed as proof of payment.
- An additional registration fee is paid to the testing vendor.
-
Qualifications
In order to be certified as a Medication Assistant-Certified (MA-C) in Arkansas, the following must be submitted to the Arkansas State Board of Nursing:- Completion of an Arkansas approved medication assistant training program (a minimum of 100 classroom and clinical hours) or has completed a portion of a nursing education program equivalent to the Medication Assistant Training course;
- Completion of an approved program is validated by submission of the Certificate of Completion form from the Program Director/Chairman. This form is certification from the Program Director or Chairman to verify a student's qualifications and graduation status after they have completed the program. It includes that the individual
- Is currently listed in good standing on the state's nurse aide registry;
- Has maintained registration on the state's certified nurse aide registry continuously for a minimum of one (1) year;
- Has completed at least one (1) continuous year of full-time experience as a certified nurse aide in this state;
- Is currently employed at a designated facility;
- Has a high school diploma or the equivalent;
- Has successfully completed a literacy and reading comprehension screening process approved by the Board; and
- Has successfully completed a medication assistant certified training course approved by the Board;
- Completion of an approved program is validated by submission of the Certificate of Completion form from the Program Director/Chairman. This form is certification from the Program Director or Chairman to verify a student's qualifications and graduation status after they have completed the program. It includes that the individual
- Passing scores from a medication assistant certification examination administered by the testing vendor D & S Diversified Technologies.
- Completion of an Arkansas approved medication assistant training program (a minimum of 100 classroom and clinical hours) or has completed a portion of a nursing education program equivalent to the Medication Assistant Training course;
-
Registration for testing
- Register for Medication Assistant – Certified testing by accessing D & S Diversified Technologies website at http://hdmaster.com and completing the Arkansas CMA Medication Assistant Exam.
- The medication assistant certified training program that you completed should provide you with the school’s five-digit program code.
-
Medication Assistant-Certified (MA-C) Endorsement Application (required for individuals that have completed Medication Assistant Certified training from another state and are applying for MA-C in Arkansas):
- This Application is for an individual that has completed Medication Aide Certified (MA-C) training from another state and is making an application for MA-C in Arkansas.
- It is NOT an application for an Arkansas MA-C renewal, an Initial MA-C exam, or any type of nursing application.
- The information contained herein is designed to assist you with the information needed to begin the application process. Read the instructions and provide all required documentation. Additional instructions are located within the online system as you progress through the application process.
- Click here for additional information located on our website at www.arsbn.org.
- General Instructions
- Application Information
- ASBN Application
- After completing all fields on a page click the “Save and Continue” button to proceed to the next page or click the “Save and Return to Home” button if you need to exit the application and return at a later time.
- If you need to change information entered in an area, you are provided an opportunity to edit information prior to submitting the application.
- No application is complete until submitted and all required documentation and fees are received. Completed applications are valid for one year from the date of submission.
- If you are required to submit any documents, upload them through your Arkansas Nurse Portal.
- ASBN Application
- Verification Form
- Submit the verification form of the original certification. Click here to download the form.
- Fee Information
- Click here for current fees.
- The accepted method of payment is with a credit card (Visa, MasterCard, or Discover).
- Verify that the charges are correct before submitting an application.
- ALL FEES ARE NON-REFUNDABLE.
- You will receive a payment receipt by email which should be printed as proof of payment.
- Qualifications
In order to be certified as a Medication Assistant-Certified (MA-C) in Arkansas, you must submit to the Arkansas State Board of Nursing written evidence you:- Have a high-school diploma or equivalent;
- Completion of a medication assistant training program substantially similar to Arkansas approved programs (a minimum of 100 classroom and clinical hours);
- Pass a medication assistant certification examination substantially similar to the Arkansas exam.
- Employer Verification
- Submit the Verification of Employment form to your most recent nursing home employer for completion. Click here to download the form. This form validates that you:
- Have maintained registration on the state's certified nurse aide registry for a minimum of one year and you are currently listed in good standing on the registry;
- Have completed at least one (1) continuous year of full-time experience in a nursing home.
- Submit the Verification of Employment form to your most recent nursing home employer for completion. Click here to download the form. This form validates that you:
- Verification Form
- Submit the verification form of original certification (Click here to download form) to the certifying agency in the state where you were originally certified. Supply your full name, current address, and original certification number so that your records can be readily located. The certifying agency will complete the form and return it directly to the ASBN office (The certifying agency may charge a fee for this service).
- MA-C Scope of Practice course
- Prior to being endorsed, persons seeking endorsement must complete the Board-approved Medication Assistant-Certified Scope of Practice course and score a minimum of 80% on a post-test related to the contents of the medication assistant chapter of the Arkansas State Board of Nursing’s Rules. Contact ASBN through the message center regarding the post-test.
- Application Information
Applications
Resources
- Instructions and Notification of Intent for Use of Medication Assistants-Certified in Nursing Homes
- CE Broker Information
- MA-C Application Process Checklist
- MA-C Verification of Certification – Endorsement (if you're already a MA-C in another state, send this form to the Board that issued the original MA-C)
- MA-C Verification of Employment Form (if you're already a MA-C, send this form to employer)
- MA-C Verification of Equivalency (form for nursing students requesting to be a MA-C)
- MA-C Verification of Program Completion (form for a student who has completed MA-C program)
ASBN - Insulin & Glucagon Administration Training Programs
Pursuant to Section 504 of the Rehabilitation Act of 1973, as it existed on July 1, 2011, the 2011 Arkansas General Assembly passed a law pertaining to the administration of glucagon by trained volunteer school personnel to Arkansas public school students who are diagnosed with diabetes. Further, the 2015 Arkansas General Assembly passed a law pertaining to the administration of insulin by trained volunteer school personnel to Arkansas public school students who are diagnosed with diabetes.
These laws allow trained volunteer school personnel in the absence of the school nurse to administer glucagon, insulin or both to a child diagnosed with diabetes in an emergency situation. Consequently, the Arkansas Department of Education and the Arkansas State Board of Nursing, in collaboration with the Arkansas School Nurses Association and diabetic experts, worked together to identify and approve Insulin and Glucagon Administration Training programs that meet the requirements of the Arkansas State Board of Nursing Rules, Chapter 9.
Approved Training Programs
- Glucagon Administration Training Program(s)
- Insulin Administration Training Program(s)
- Insulin and Glucagon Administration Training Program(s)
Certifications of Training
- Certification of Training in Glucagon Administration for Volunteer Personnel in Schools (Skill Checklist) - template sample
- Certification of Training in Insulin Administration for Volunteer Personnel in Schools (Skill Checklist) - template sample
Certificates of Approval
- Certificate of Approval - Glucagon Administration Training Program - National Diabetes Education Program
- Certificate of Approval - Insulin Administration Training Program - National Diabetes Education Program
- Certificate of Approval - Insulin, and Glucagon Administration Training Program - National Diabetes Education Program
Certificates of Participation